Evaluate risk factors and clinical outcomes of infections caused by Enterobacteriaceae resistant to third-generation cephalosporins present in samples collected upon hospital admission.
MethodsRisk factors were evaluated using a 1:2 ratio case–control study. Influence of resistance on the appropriateness of antibiotic therapy, length of stay, and hospital mortality were prospectively evaluated. Characteristics independently associated with the presence of resistant enterobacteria were assessed by logistic regression.
ResultsEnterobacteria resistant to third-generation cephalosporins were quite common (26.0%). Male gender (OR: 2.66; 95% CI, 1.17–5.06; p=0.019), invasive prosthesis (OR: 3.79; 95% CI, 1.29–11.08; p=0.015), previous use of cephalosporins (OR: 2.77; 95% CI, 1.10–6.97; p=0.029) and hospitalization in the last 6 months (OR: 5.33; 95% CI, 2.29–12.44; p<0.001) were independently associated with the presence of these microorganisms. These bacteria were associated with higher frequency of inappropriate antimicrobial therapy, worse clinical response, and longer length of stay. Finally, older age, admission to the ICU, and site of infection other than urinary tract were independently associated to higher hospital mortality.
ConclusionsRisk factors identified in this study may help in the choice of empirical antibiotic therapy for infected patients suspected of harboring these bacteria and in the early implementation of measures to avoid the spread of these bacteria in the hospital environment.
Enterobacteriaceae are responsible for a wide variety of nosocomial and community-acquired infections.1,2 Given their versatility, low toxicity, and broad spectrum of action, β-lactam antibiotics, such as the third generation cephalosporins, are the main choice for the treatment of infections caused by these microorganisms.3 However, along with their overuse, a decrease in the effectiveness of these compounds has been observed in recent years.4 In Enterobacteriaceae, antibiotic resistance due to the production of extended-spectrum β-lactamase (ESBL-E) and overexpression of AmpC cephalosporinase (AmpC-E) is cause for great concern and produce a significant impact both on empirical and definitive therapy.5,6 Moroeover, this resistance may lead to delays in the onset of effective antimicrobial therapy, with consequent impact on clinical results, and higher mortality rate.7
Many studies have attempted to determine the risk factors for nosocomial infections caused by Enterobacteriaceae resistant to third-generation cephalosporins, and more recently, some publications have addressed those cases identified at the time of hospital admission.7–9 Our aim was to determine the frequency, risk factors, and the impact on clinical outcome of the presence of Enterobacteriaceae resistant to third-generation cephalosporins isolated in samples collected within the first 48h of hospitalization of patients admitted to a university hospital.
Material and methodsStudy setting and subjectsThis two-phase study was conducted from August 2011 to July 2012, in a 501-bed University Hospital of Minas Gerais, Brazil. Firstly, a 1:2 ratio case–control protocol was run, in order to identify characteristics associated with colonization or infection by resistant Enterobacteriaceae in samples obtained during the first 48h of hospitalization. Thereafter, in the second phase of the study, the included patients were followed up in order to identify the impact of these microorganisms in some clinical endpoints. Patients at least 18 years old, whose culture requested by the attending physician grew enterobacteria in samples collected in pre-specified days after hospitalization were assessed for potential inclusion. Patients with enterobacteria resistant to carbapenems were excluded from the study. All included patients lived in the metropolitan area of Belo Horizonte, Minas Gerais state.
Eligible patients were screened in the emergency service of our hospital and categorized into one of two groups: (i) cases, referring to those colonized or infected by Enterobacteriaceae resistant to third-generation cephalosporins and, (ii) controls, patients colonized or infected by Enterobacteriaceae susceptible to third-generation cephalosporins. For each case included in the study, two controls were selected sequentially on the same day. Each patient was included in the study only once.
Patients were further categorized as (i) infected patients, if there was a clinical suspicion of active infection leading to prescription of antibiotic therapy, and (ii) colonized patients, if there was no clinical suspicion of infection or if the enterobacteria were isolated from surveillance samples. Surveillance samples were samples collected at the time of hospital admission via swabs, as a routine procedure in patients transferred from other hospitals aiming at detecting the presence of patients colonized with multidrug-resistant bacteria.
Study procedures and definitionsDemographic, clinical, and epidemiological data were collected using a dedicated case report form. Data was obtained from interviews and by consulting electronic and printed records. The following variables were collected: age, sex, microbiological data, site of infection, presence of comorbidities (diabetes, chronic renal failure, liver failure, solid tumor, malignant hematological disease, heart failure, and others), known immunosuppression (HIV infection, neutropenia with PMN <500cells/mm3, use of corticosteroids in doses above 15mg/day of prednisone or equivalent, use of other immunosuppressive drugs), hospitalization and previous use of antibiotics, performance of invasive procedures in the last four weeks, use of invasive prosthesis, presence of stoma, recurrent urinary tract infection (UTI), adequacy of initial empiric antibiotic treatment (only for infected patients), hospitalization at the intensive care unit (ICU), response to the antibiotic therapy, all-cause hospital mortality, and hospital length of stay.
Recurrence of urinary tract infection (UTI) was defined as the development of two or more documented episodes in the last six months. For the subgroup of infected patients, empirical treatment was considered adequate when an antimicrobial regimen included an active antibiotic against the isolated enterobacteria, and was initiated at the recommended dose in the first 24h after sample collection. Inadequate antimicrobial treatment included absence of antimicrobial agents indicated for a specific class of microorganisms and administration of an antimicrobial agent to which the isolated microorganism was resistant.10
In this subset of patients, clinical response to the antimicrobial treatment was classified as: “complete or partial resolution”, referring to the patients who presented total or partial improvement of fever, leukocytosis and clinical signs of infection, and “therapeutic failure or uncertain result”, for those who, respectively, showed no decrease in these parameters at all or persisted with symptoms and signs that were not clearly attributable to infection.11
For the infected patients clinical outcomes were ICU admittance during follow-up, all-cause hospital mortality, and length of hospital stay.
Microbiological analysisBacterial identification and antimicrobial susceptibility testing, including production of β-lactamases, were carried out in accordance with the recommendations of the Clinical and Laboratory Standards Institute (CLSI).12 Identification and ESBL production were confirmed by using the API 20E® and Vitek II® systems (both from bioMérieux).
Statistical analysisCategorical variables were compared by using the Pearson's chi-square test (or Fischer's exact test, as indicated), and presented with the corresponding 95% confidence intervals (CI). Continuous variables were compared using Student's t-test. In cases of non-normal distribution of quantitative variables, non-parametric tests were used, such as Mann–Whitney U test.
Non-conditional multivariate logistic regression analysis was conducted to determine the independent risk factors associated with the presence of resistant enterobacteria. All variables presenting the p-value <0.20 in univariate analysis were included in the multivariate model. Furthermore, the independent risks factors associated with in-hospital death were investigated in a multivariate analysis excluding the colonized patients. Using the same criteria described above (p-value <0.20 in the univariate analysis), the following variables were include in this analysis: age, presence of two or more comorbidities, presence of UTI, admittance to ICU, inappropriate empirical antibiotic therapy, and presence of resistant enterobacteria (cases). For the multivariate models, backward method was used, and the Hosmer and Lemeshow test was applied to assess the models’ adequacy.
Finally, to evaluate the impact of the infections caused by enterobacteria resistant to third-generation cephalosporins on hospital length of stay, a Cox proportional hazards analysis was conducted. The variable “age” was included as a binary variable (greater than or less than 50 years). Discharges by death were censored. For all analyses, a two-tailed value of p<0.05 was considered significant. Statistical analyses were performed by using SPSS version 16.0 software.
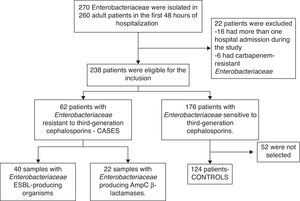
ResultsSelection of studied patientsDuring the study period, 238 adult patients had one or more Enterobacteriaceae isolated from samples collected in the first 48h after hospital admission. Of this total, 62 (26.0%) patients had Enterobacteriaceae resistant to third-generation cephalosporins. The flowchart presenting the inclusion procedures is summarized in Fig. 1.
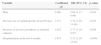
Demographic characteristics and comorbiditiesResults of univariate analysis are shown in Table 1. Case patients had higher mean age (56.7±19.1 vs. 46.9±19.6, p=0.001), were more frequently male (53.2% vs. 25.8%; p<0.001), and usually presented two or more comorbidities (50.0%vs. 34.7%, p=0.044). Specifically, heart failure (HF) was the only comorbidity significantly more frequent among cases (17.7% vs. 7.3%, p=0.031).
Demographic and clinical characteristics of patients in whom there was isolation of Enterobacteriaceae in samples collected in the first 48h of hospitalization.
| Variables | Casesn=62 | Controlsn=124 | p-value | OR (95% CI) |
|---|---|---|---|---|
| Male,n(%) | 33 (53.2) | 32 (25.8) | <0.001 | 3.27 (1.72–6.21) |
| Age, years, mean (SD) | 56.7 (19.1) | 46.9 (19.6) | 0.001 | |
| Comorbidities | ||||
| Diabetes mellitus, n (%) | 14 (22.4) | 24 (19.4) | 0.607 | 1.22 (0.58–2.56) |
| Chronic renal failure, n (%) | 3 (4.8) | 4 (3.2) | 0.586 | 1.53 (0.33–7.04) |
| Liver failure, n (%) | 3 (4.8) | 3 (2.4) | 0.379 | 2.05 (0.40–10.47) |
| Solid Tumor, n (%) | 22 (35.5) | 36 (29.0) | 0.371 | 1.34 (0.70–2.57) |
| Malignant hematological disease, n (%) | 3 (4.8) | 4 (3.2) | 0.586 | 1.53 (0.33–7.04) |
| Heart Failure, n (%) | 11 (17.7) | 9 (7.3) | 0.031 | 2.73 (1.07–6.99) |
| Presence of 2 or more comorbidities | 31 (50.0) | 43 (34.7) | 0.044 | 1.88 (1.01–3.50) |
| Coexisting conditions | ||||
| Transplant | 4 (6.5) | 10 (8.1) | 0.694 | 0.79 (0.24–2.61) |
| Immunosuppression, n (%) | 19 (30.6) | 30 (24.2) | 0.346 | 1.38 (0.70–2.73) |
| Immunosuppressive drugs, n (%) | 16 (25.8) | 26 (21.0) | 0.457 | 1.31 (0.64–2.68) |
| HIV, n (%) | 2 (3.2) | 2 (1.6) | 0.475 | 2.03 (0.28–14.79) |
| Primary site of infection | <0.001 | |||
| Urinary tract, n (%) | 37 (59.7) | 103 (83.1) | ||
| Respiratory tract, n (%) | 2 (3.2) | 4 (3.2) | ||
| Bloodstream, n (%) | 2 (3.2) | 6 (4.8) | ||
| Skin and soft tissue, n (%) | 6 (9.7) | 6 (4.8) | ||
| Other sites, n (%) | 6 (9.7) | 5 (4.0) | ||
| Severity on admission (Manchester) | ||||
| Orange or red risk rating, n(%) | 21 (33.9) | 38 (30.6) | 0.656 | 1.16 (0.61–2.22) |
Taking into account the whole sample of patients, there was a predominance of infected patients (81.7%); however, there was significantly more colonized individuals among cases as compared to controls (25.8% vs. 12.9%, P=0.028). Out of all cases, 14.5% were detected through surveillance cultures. For both groups, UTI was the most common site of infection (83.1% in the control group vs. 59.7% in cases, p<0.001). A significant difference was observed in the frequency of species of Enterobacteriaceae between the groups (p<0.001). Escherichia coli was the most frequently species isolated from the controls (79.0%) whereas in the cases prevailed Klebsiella pneumoniae (30.6%) with E. coli (30.6%). Enterobacter sp. and Serratia marcescens were isolated only from cases (24.2% and 6.0%, respectively).
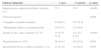
Risk factors for Enterobacteriaceae resistant to third-generation cephalosporinsThe univariate analysis testing the variables associated with the presence of Enterobacteriaceae resistant to third-generation cephalosporins is summarized in Table 2. The composite presence of invasive prosthesis or tunneled catheters and presence of stoma were significantly more frequent among cases than controls. The main types of stoma observed in these patients were cystostomy (41.2%), colostomy (23.5%), and tracheostomy (17.6%). Finally, previous contact with health care services (performance of invasive procedures and/or prior hospitalization) and previous use of antibiotics were also more frequent in patients with resistant Enterobacteriaceae.
Univariate analysis of risk factors for patients with isolation of Enterobacteriaceae resistant to third-generation cephalosporins in the first 48h of hospitalization.
| Risk factor | Casesn=62 | Controlsn=124 | p-value | OR (95% CI) |
|---|---|---|---|---|
| Patient transferred from another hospital,n(%) | 10 (16.4) | 14 (11.3) | 0.331 | 1.54 (0.64–3.70) |
| Invasive prosthesis or tunneled catheters,n(%) | 24 (44.4) | 21 (17.4) | <0.001 | 3.81 (1.87–7.78) |
| Stoma | 11 (19.0) | 6 (4.8) | 0.002 | 4.60 (1.61–13.16) |
| Recurrence of UTI | 18 (35.3) | 27 (22.7) | 0.088 | 1.86 (0.91–3.81) |
| Previous contact with health care services | ||||
| Invasive procedures | 24 (40.7) | 20 (16.3) | <0.001 | 3.53 (1.74–7.16) |
| Prior hospitalization in the last 6 months | 33 (60.0) | 24 (20.3) | <0.001 | 5.88 (2.91–11.85) |
| Prior hospitalization in ICU in the last 6 months | 12 (22.6) | 11 (9.3) | 0.018 | 2.85 (1.17–6.96) |
| Previous use of antimicrobials | ||||
| Last 90 days | ||||
| All | 39 (73.6) | 56 (47.1) | 0.001 | 3.13 (1.54–6.37) |
| Cephalosporins | 15 (28.3) | 14 (11.8) | 0.007 | 2.96 (1.31–6.710) |
| Quinolones | 11 (21.2) | 17 (14.3) | 0.264 | 1.61 (0.69–3.73) |
| β-lactam/β-lactamase inhibitor | 8 (15.1) | 10 (8.4) | 0.186 | 1.94 (0.72–5.23) |
| Last 12 months | 38 (76.0) | 41 (43.6) | <0.001 | 4.09 (1.90–8.81) |
Previous use of cephalosporins in last 90 days, presence of invasive prosthesis or tunneled catheters, hospitalization in last six months, and male gender were independently associated with the presence of Enterobacteriaceae resistant to third generation cephalosporins as shown in Table 3.
Independent risk factors for the isolation of Enterobacteriaceae resistant to third generation cephalosporins in samples collected in the first 48h of hospitalization.
| Variable | Coefficient (β) | OR (95% CI) | p-value |
|---|---|---|---|
| Male | 0.980 | 2.66 (1.17–6.06) | 0.019 |
| Previous use of cephalosporins in last 90 days | 1.333 | 3.79 (1.29–11.08) | 0.015 |
| Presence of invasive prosthesis or tunneled catheters | 1.022 | 2.77 (1.10–6.97) | 0.029 |
| Hospitalization in the last 6 months | 1.675 | 5.33 (2.29–12.44) | <0.001 |
We prospectively tested the impact of the presence of enterobacteria resistant to third-generation cephalosporins on some clinical endpoints (Table 4). Inappropriate empirical antibiotic therapy was significantly higher among cases (73.3% vs. 10.3%, p<0.001). Moreover, patients with resistant Enterobacteriaceae were more often admitted to the ICU (p=0.003), had worse clinical response to the antimicrobial therapy (p=0.029), and had higher length of stay (p<0.001). Although the death rate was higher among cases (21% vs. 10.5%), this difference did not quite reach statistical significance (p=0.052).
Clinical impact of the presence of Enterobacteriaceae resistant to third generation cephalosporins in samples collected within the first 48h of hospitalization.a
| Clinical endpoints | Cases | Controls | p-value |
|---|---|---|---|
| Inappropriate empirical antibiotic therapy (%) | 73.3 | 10.3 | <0.001 |
| Clinical response | 0.029 | ||
| Complete or partial resolution | 30 (62.5) | 87 (79.1) | |
| Therapeutic failure or uncertain result | 18 (37.5) | 23 (20.9) | |
| Length of stay, days, median (25–75) | 13 (4.75–31.0) | 5 (1.25–9.75) | <0.001 |
| Hospitalization in UCI | 26 (44.1) | 28 (22.6) | 0.003 |
| Hospital discharge condition (death), n (%) | 13 (21.0) | 13 (10.5) | 0.052 |
As shown in Table 5, six variables were associated with death during hospitalization in univariate analysis, but seven variables reached criteria to be included in the multivariate model (p<0.20). From these, only increasing age, hospitalization at the ICU, and a primary site of infection other than UTI proved to be independently associated to all-cause hospital mortality.
Factors associated with death during hospitalization.
| Variable | Deceased | Survivors | p-valueUnivariate | OR (95% CI) | p-valueMultivariate |
|---|---|---|---|---|---|
| Age, years, average (SD) | 62.1 (17.8) | 48.7 (20.0) | 0.002 | 1.03 (1.00–1.05) | <0.001 |
| Presence of 2 or more comorbidities (%) | 60.0 | 36.9 | 0.041 | NS | NS |
| Infection site other than UTI | 82.2 | 56.0 | 0.007 | 3.20 (1.12–9.26) | 0.030 |
| Immunosuppression | 36.0 | 25.6 | 0.284 | NS | NS |
| Admission to ICU | 72.0 | 18.8 | <0.001 | 8.53 (3.08–23.66) | <0.001 |
| Inappropriate empirical antibiotic therapy (%) | 48.0 | 25.2 | 0.022 | NS | NS |
| Enterobacteriaceae resistant to third generation cephalosporins (cases) (%) | 48.0 | 26.4 | 0.030 | NS | NS |
| Enterobacteriaceae other than E. coli | 56.0 | 24.9 | 0.059 | NS | NS |
NS, non-significant.
Finally, to be older than 50 years (HR: 1.63; 95% CI: 1.18–2.27; p=0.003) and presence of Enterobacteriaceae resistant to third generation cephalosporins (HR: 2.31; 95% CI: 1.59–3.34; p<0.001) were independently associated with longer length of stay.
DiscussionIn this study, we demonstrated that the presence of Enterobacteriaceae resistant to third generation cephalosporins was quite common (26%) in cultures obtained in the first 48h of hospitalization of adult patients admitted to a university hospital in Brazil. The presence of invasive prosthesis or tunneled catheters, previous use of cephalosporins, hospitalization in the last six months, and be male proved to be risk factors independently associated with the presence of these bacteria. Also, the presence of resistant enterobacteria was associated with inadequate empirical antibiotic therapy and longer hospital stay.
In recent years, the frequency of Enterobacteriaceae resistant to third generation cephalosporins has increased and disseminated in the hospital environment4,13–15 and more recently in non-hospital settings.5,6,16,17 In Brazil, most studies conducted up to now determined the frequency and risk factors associated with these pathogens in patients with nosocomial infections.18,19 The frequency of Enterobacteriaceae resistant to third generation cephalosporins in samples obtained during the first 48h of hospitalization found in this study was considerably high. The rate found in this study is in line with other reports of nosocomial infection rates in small Brazilian hospitals.20,21 In the few studies conducted in the country with outpatients the ESBL production rates were considerably lower.22,23 However, in the present study most cases had had recent hospitalizations (68.4%) or were transferred from other hospitals (16.4%), so they could not be considered as community-acquired. Rather, it seems more appropriate to consider them as health care associated infections.
Notwithstanding the country of interest, most studies have pointed to previous exposure to health care settings as risk factor for the presence of ESBL-determined resistance among Enterobacteriaceae identified in cultures obtained at the time of hospital admission.7,24,25 Similarly, this study identified previous hospitalization in the last six months, and presence of invasive prosthesis or tunneled catheters, and previous use of cephalosporins as factors independently associated with the presence of these bacteria. As for the predominance of male patients, other studies also demonstrated an increased frequency of ESBL-producing Enterobacteriaceae among men,9,24,26,27 but nonetheless this issue remains controversial.8,28,29 According to Behar30 the discrepancies are related to methodological differences between the studies, especially the selection of the control group, as well as differences in infection presentation and antibiotics prescribing patterns between genders (e.g., women have UTI more often).
In this study, the presence of resistant Enterobacteriaceae was associated with poor clinical outcomes, such as longer length of hospital stay, more frequent hospitalizations at the ICU, and worse clinical response to antibiotic therapy. However, this finding should be interpreted with caution since patients with resistant enterobacteria had higher mean age and more comorbidities. Inadequate antibiotic therapy proved to be significantly more common among cases (73.3% vs. 10.3%), and is one of the possible explanations for less favorable outcomes observed among patients with resistant enterobacteria. Delay to administer appropriate antibiotic therapy correlated with a worse prognosis in several clinical conditions.26,31,32 Finally, regarding hospital stay, previous reports have shown longer length of stay of patients with infections caused by resistant Gram-negative bacilli (GNB) when compared to infections caused by sensitive GNB.33
This study has several limitations. First, it is a single center study, involving a relatively small sample size (186 subjects), which might not be representative of the overall patient population admitted to other hospitals in Brazil or elsewhere. Thus, our findings must be validated in other cohorts, both inside and outside Brazil. The characteristics of the patients studied here prevent us from stating that the resistant enterobacteria isolated in our patients were community-acquired, being more accurate to consider them as associated with health care facilities. Finally, we evaluated ESBL producers and strains overexpressing AmpC as a single group of enterobacteria resistant to third-generation cephalosporins. The small number of isolates prevented us from performing an analysis separating these two subgroups.
In conclusion, in the present study, consisting of adult patients admitted to a university hospital of high complexity in Brazil, the occurrence of enterobacteria resistant to third-generation cephalosporins is quite common. The recognition of risk factors associated with the presence of these bacteria may have an impact on the choice of empirical antibiotic therapy, particularly in patients with organ dysfunction.
Conflicts of interestThe authors declare no conflicts of interest.
Ethics statementThe study was approved by the Ethics Committee of the institution – COEP UFMG (CAAE-0211. 0.203.000-11) – and has been performed in accordance with the ethical standards laid in the 1964 Declaration of Helsinki and its later amendments. All participants provide their written informed consent to participate.
We strongly appreciate the support given by the Microbiology Laboratory's team from Hospital das Clínicas of UFMG, without which this work would not have been possible.
This study was partially supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG (APQ-00823-11).