Aeromonas species are renowned enteric pathogens with virulence determinants linked to human diseases, such as gastroenteritis, skin, soft-tissue and muscle infections, and septicemia. A recent concern of resistance in this organism has emerged, especially the presence carbapenemases. Herein we describe a case series of emerging carbapenem-resistant Aeromonas species infection in our hospital in Cali, Colombia.
Materials and methodsCases from 2012 to 2018 are reported. Clinical data was abstracted from the clinical charts and laboratory information. Phenotypic detection of resistance was identified using the VITEK®2 system (BioMérieux) and broth microdilution MicroScan WalkAway plus System (Beckman Coulter). CARBA NP-test and multiplex qPCR assay was performed in 11 isolates to identify genes encoding carbapenemases (blaKPC, blaVIM, blaIMP and blaNDM).
Results21 cases of Aeromonas infection in hospitalized patients with phenotypic resistance to carbapenems were studied. The median age was 50 years, 55% (12/21) were male, and 67% (14/21) were healthcare-associated infections (HAI). Aeromonas hydrophila was the most common species (19/21). Forty-three percent (9/21) of the patients were immunocompromised. The mortality was 33% (7/21), and in patients with bacteremia was 100%. Most patients received empirical treatment with meropenem and failed to this treatment. PCR amplification tests showed negative results for the carbapenemases analyzed.
ConclusionEmerging phenotypic carbapenem-resistant infection has been seen in our hospital, most as HAI. High mortality was found, especially in immunocompromised patients and in those who failled empirical treatment with carbapenems. As the main carbapenemases tested were negative, carbapenem-resistant could be attributed to an intrinsic metallo-β-lactamase, CphA encoded by the cphA gene, possible hyperproduction of ampC β-lactamase and/or porins expression.
Aeromonas spp. are Gram-negative, oxidase-positive, rod-shaped bacteria, mainly found in marine environments and freshwater.1,2 The incidence and survival of the bacterium are affected by the pH, temperature, salt content and dissolved oxygen in the water.3 Their transmission is related to contact and ingestion of contaminated water or food. Aeromonas infection has also been associated with reptile bites and water-related activities like fishing and diving.4
The global incidence of Aeromonas infections is unknown due to underreporting. The first state in the United States to report infections with this germ was California in 1988, where 219 patients were analyzed, showing an incidence of 10/1,000,000 people. Another study in France reported a prevalence of 1.6 infections in one million people.5
Aeromonas infections have a wide range of clinical manifestations, like skin and soft tissue infections, hepatobiliary diseases, diarrhea, and even bacteremia. Previous studies reported a mortality rate of 30% due to this infections.6
Aeromonas spp. resistant to certain antibiotics like penicillins have been broadly described.7 However, there are few data regarding carbapenems resistance, which has been associated with the production of “Carbapenems hydrolyzing Aeromonas” (CphA). Carbapenems use as an empiric treatment in severe infections is increasing, and some guidelines indicate the use of these antibiotics for the treatment of skin and soft tissue infections related to water.6
This study aimed to describe the clinical course of phenotypically carbapenems-resistant Aeromonas spp. infection and the presence of phenotypic resistance without an associated gene.
Materials and methodsA cross-sectional study was conduted at Fundación Valle del Lili, a University hospital, and referral center for the southwest of Colombia. This study was approved by the institutional review board (IRB) under protocol No. 1019.
Patients with carbapenems-resistant Aeromonas spp. infections were included from January 2012 to September 2018. The medical data were obtained from clinical charts and microbiology results from the Fundación Valle del Lili laboratory.
Laboratory methodsAll the samples were plated on blood agar and McConkey agar for bacterial isolation. The isolates were identified using the VITEK®2 system (BioMérieux), and susceptibility tests were performed by broth microdilution using the MicroScan WalkAway plus System (Beckman Coulter). Isolates were classified as resistant to carbapenems (Imipenem, Meropenem, and Doripenem) according to the breakpoints of the CLSI (Clinical and Laboratory Standards Institute) M45 document guidelines for that year.8 Carbapenemase phenotypic tests were not performed in the laboratory. The isolates were stored in skim milk for further analysis. Eleven isolates were recovered from the skim milk and subsequently sent to CIDEIM (Centro Internacional de Entrenamiento e Investigaciones Médicas) for molecular analysis. CARBA NP-test9 and multiplex qPCR assay were performed in the isolates recovered to identify genes encoding carbapenemases (blaKPC, blaVIM, blaIMP and blaNDM) as previously described at CIDEIM.10
Statistical analysisContinuous variables were presented as median and interquartile ranges. Categorical variables were presented in frequencies and percentages. Carbapenems-resistant Aeromonas infection mortality was calculated as a proportion between deceased patients with phenotypically carbapenems-resistant Aeromonas spp. and patients with positive cultures for Aeromonas spp. Statistical analyses were performed in STATA® (StataCorp, 2011, Stata 12 Base Reference Manual, College Station, TX: StataPress).
ResultsOut of 79 inpatients with Aeromonas species infections identified, 21 showed resistance to carbapenems. Of those, the median age was 50 years (IQR = 29–67 years), and 12 patients were male (57%). Regarding possible sources of exposure to Aeromonas spp., four patients had probable occupational exposure, such as water contact (Table 1).
Demographic and epidemiological characteristics of included patients (n = 21).
| Characteristics | Cases, n = 21 n (%) |
|---|---|
| Sex | |
| Male | 12 (57) |
| Age (years)a | 50 (29–67) |
| Occupation | |
| Fisherman | 1 (5) |
| Farmer | 1 (5) |
| Military forces | 2 (10) |
| Other | 4 (19) |
| Unknown | 13 (62) |
| Previous water contact | 4 (19) |
| Mode of infection | |
| Invasive medical device | 6 (29) |
| Surgical wound infection | 4 (19) |
| Foodborne transmission | 3 (14) |
| Organic elements exposure | 3 (14) |
| Trauma with water contact | 1 (5) |
| NA | 4 (19) |
| Comorbidities | |
| Immunosuppression | 9 (43) |
| Cardiovascular disease | 5 (24) |
| Hepatic disease | 3 (14) |
| Solid organ transplant | 3 (14) |
| Hematologic malignancy | 3 (14) |
| Chronic renal disease | 2 (10) |
| Diabetes | 2 (10) |
| Solid organ tumor | 2 (10) |
| Neurologic disease | 2 (10) |
| Rheumatologic disease | 2 (10) |
| HIV/AIDS | 1 (5) |
| Antibiotic use history | 6 (29) |
| Recent surgery (≤3 months) | 8 (38) |
NA: not available.
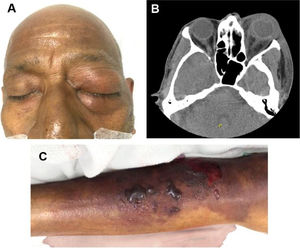
The main comorbidity was cardiovascular disease (24%). Forty-three percent were immunosuppressed, secondary to concomitant clinical conditions or medications. Sixty-seven percent (n = 14) had a healthcare-associated infection (HAI). The main sources of infection were invasive medical devices (such as central venous catheters, osteosynthesis material, abdominal catheters, among others) in six (28.6%) patients and surgical wound infection in four (19%) patients, all of them with HAI. Among patients with community-acquired infections (CAI), the main source of infection was previous exposure to organic material in three (43%) patients. Table 2 shows the main characteristics associated with HAI and CAI. Fig. 1 shows an ocular and lower extremity Aeromonas infection.
Clinical characteristics and outcomes of included patients (n = 21).
| Total, n = 21 n (%) | Type of infection | ||
|---|---|---|---|
| HAI, n = 14 n (%) | CAI, n = 7 n (%) | ||
| Sex | |||
| Male | 12 (57) | 6 (43) | 6 (86) |
| Agea | 50 (29–67) | 49 (28–64) | 49 (23–71) |
| Classification of Aeromonas | |||
| A. hydrophila | 19 (90) | 12 (86) | 7 (100) |
| A. sobria | 2 (10) | 2 (14) | 0 |
| Sample type | |||
| Blood culture | 5 (24) | 4 (29) | 1 (14) |
| Stool culture | 5 (24) | 3 (21) | 2 (29) |
| Peritoneal lavage | 5 (24) | 3 (21) | 2 (29) |
| Surgical wound discharge | 1 (5) | 1 (7) | 0 |
| Skin wound discharge | 2 (10) | 1 (7) | 1 (14) |
| Bone and wound discharge | 1 (5) | 0 | 1 (14) |
| Lung decortication | 1 (5) | 1 (7) | 0 |
| Bile | 1 (5) | 1 (7) | 0 |
| Resistance to second-generation cephalosporins | 16 (76) | 11 (79) | 5 (71) |
| Resistance to third-generation cephalosporins | 7 (33) | 6 (43) | 1 (14) |
| Carbapenem-resistance profile | |||
| Meropenem resistance | 8 (38) | 6 (43) | 2 (29) |
| Imipenem resistance | 4 (19) | 3 (21) | 1 (14) |
| Meropenem and imipenem resistance | 8 (38) | 5 (36) | 3 (43) |
| Ertapenem resistance | 1 (5) | 0 | 1 (14) |
| Use of invasive devices | 11 (52) | 9 (64) | 2 (29) |
| ICU hospitalization | 15 (71) | 11 (79) | 4 (57) |
| Length of ICU hospitalization (days)a | 12 (4–43) | 12 (4–43) | 20.5 (4–86.5) |
| Vasoactive support | 10 (48) | 6 (43) | 4 (57) |
| Inotropic support | 7 (33) | 5 (36) | 2 (29) |
| Ventilatory support | 10 (48) | 7 (50) | 3 (43) |
| Multiple organ failure | 10 (48) | 8 (57) | 2 (29) |
| Surgical management | 9 (43) | 4 (29) | 5 (71) |
| Outcomes | |||
| Recovery | 14 (67) | 10 (71) | 4 (57) |
| Death | 7 (33) | 4 (29) | 3 (43) |
HAI: hospital acquired infection; CAI: community-acquired infection; ICU: intensive care unit; NA: not available.
Ocular and lower member Aeromonas infection. (A) Postseptal cellulitis by carbapenem-resistant Aeromonas infection. (B) Axial CT scan showing an increased volume and density of the left periorbital soft tissues associated with ocular proptosis and enhancement of the orbital septum. Alteration in intraconal fat density with laminar fluid in the posterior and medial region of the eyeball. (C) Soft-tissue infection by carbapenem-resistant Aeromonas in the right lower limb.
Most Aeromonas spp. were isolated from blood cultures (25%), 19 were Aeromonas hydrophila (90%) and two were Aeromonas sobria. CARBA NP was negative in 10 out of 11 isolates analyzed and qPCR detection of carbapenem-resistant genes (blaKPC, blaVIM, blaIMP and blaNDM) was negative in all isolates.
Seventy-nine percent had meropenem-resistant Aeromonas with a minimum inhibitory concentration (MIC) ≥16 μg/mL and three patients had imipenem-resistant Aeromonas HAI. Among patients with CAI, two patients had meropenem resistant Aeromonas, one was resistant to imipenem, and one patient to ertapenem.
Bacteremia was found in five patients, four with HAI and one patient with CAI. Regarding management, 15 (71%) patients required hospitalization at the intensive care unit (ICU), mean length of stay 3 days (IQR = 4–82 years). Most patients received empirical treatment with meropenem (n = 11) and seven cases failed to this treatment. The remaining cases were treated with combinations of quinolones and aminoglycosides.
All-cause mortality was 33% (n = 7); the mortality related to carbapenem-resistant Aeromonas infection was 19%. Three patients had an associated co-infection, one patient had Pseudomonas aeruginosa urinary tract infection, other patient had disseminated histoplasmosis, and another had polymicrobial soft tissue infection with Enterococcus faecalis, Enterococcus faecium, Escherichia coli and Pseudomonas mendocina, later complicated with ventilator-associated pneumonia. One patient died due to HIV/AIDS complicated with disseminated histoplasmosis and respiratory failure. All patients with bacteremia had a previous history of immunosuppression, and all of them died. The mortality among patients with CAI was 42.9% vs. 28.6% in HAI.
DiscussionThe genus Aeromonas spp. has been considered a ubiquitous microorganism. However, in recent years there has been an increase in the number of cases of infections by this genus.11 The majority of strains isolated in this study was A. hydrophila, and in smaller percentage A. sobria, in line with previous studies that showed the same species distribution.12–14Aeromonas species can express three chromosomal β-lactam-induced β-lactamases, including a group 1 molecular class C cephalosporinases, a group 2d molecular class D penicillinase, and group 3 molecular class B metallo-β-lactamase (MBL).15,16 Further, the presence of these β-lactamases in Aeromonas, in particular the carbapenemases, may not be detected by conventional susceptibility methods.17
In this study, we report 21 cases of carbapenem-resistant Aeromonas spp infection, most HAI. Resistance profile of these isolates presented meropenem, imipenem, and ertapenem resistance, but the main carbapenemase tested were negative. These cases represented 67% of total cases of Aeromonas infections in our institution, which is alarming in our context, but these infections are frequently not reported, leading to underreporting. The empirical use of carbapenems is high in our institution, due to the high prevalence of resistance through the production of extended-spectrum β-lactamases (ESBL) in our environment,18 and it is worrying that several cases (7/11) failed to treatment.
Carbapenem-resistant Aeromonas spp. has been reported previously in different countries with a prevalence between 0.5 and 7.7%. However, according to reports of the National Institute of Health in Colombia the prevalence of this microorganism is increasing since for 2015, from 8% to 10% in 2016.19 For that reason, the increase of carbapenem-resistant Aeromonas infections needs more attention.20
CphA, a metalloenzyme encoded by the cphA gene, has a very specific substrate on penem and carbapenem only, not on penicillin and cephalosporins.21 The production of CphA does not confer phenotypic resistance to imipenem or meropenem in vitro, when tested with a standard inoculum of 104–105 CFU/mL (0.5 MacFarland), but it can be shown with an increased inoculum from 108–109 CFU/mL (1.5 MacFarland). Sinclair et al. demonstrated these samples were susceptible to meropenem and imipenem by E-test, whereas by automated methods (VITEK®2) resistance was detected in 57% of cases.
CphA hydrolyzes nitrofecin poorly or not at all, indicating that the nitrocefin test is not reliable for detecting carbapenemases. In addition, CphA MBL production is not readily detected by conventional in vitro susceptibility tests with EDTA-based combination disk diffusion, E-test, or agar dilution methods with standard inocula, unless large inocula are used.22–24 Also, most Aeromonas species produce an inducible chromosomal β-lactamase, which may not be detected by rapid commercial susceptibility systems.25 Furthermore, the expression of AmpC β-lactamases includes inducible β-lactamase production in the presence of suitable inducers or development of depressed mutation, which leads to constitutive high-level production of β-lactamases.23,26 Finally, porins tend to have exclusion limits approaching the size of many antibiotics, and thus, they tend to limit the rate of diffusion of these molecules, contributing in this way to intrinsic resistance.27 Consequently, studies in this field should evaluate the intrinsic resistance of Aeromonas species to carbapenems and other broad-spectrum antibiotics, which are limited. In this sense, carbapenem resistance could be attributed to an intrinsic MBL, CphA encoded by the cphA gene, and/or possible hyperproduction of ampC β-lactamase and porins expression.
Similar to our findings, a study performed in Queensland in 2016 showed the same profile of resistance to these antibiotics by disk diffusion.6 Currently, the world seems to face the post-antibiotic era, where antibiotic resistance of bacteria is increasing.28 Therefore, the management and reasonable use of antibiotics are essential in the treatment of infections. Towards that end, the clinical use of carbapenem therapy for Aeromonas infections should be reevaluated, and carbapenem resistance tests should be implemented to perform a targeted and individualized antibiotic therapy. Studies have described phenotypic tests for carbapenemases detection with a 97.4% sensitivity and 95.7% specificity.6 In addition, other studies have showed that carbapenem monotherapy had problems with MBL inhibition.23
Predisposing factors related to comorbidities and use of immunosuppressive drugs were observed. Regarding comorbidities, cardiovascular diseases were the most frequent, followed by liver disease, solid organ transplantation, and hematological malignancy. Thus, one could propose that this type of comorbidities should be studied in subsequent researches to look for possible associations.
Aeromonas spp. infections have a high mortality rate, exceeding 30% in some researches.29,30 Our study showed that cases with fatal outcomes were related to sepsis by Aeromonas infection, HIV/AIDS co-infection, and healthcare-acquired pneumonia. Findings similar to our study were reported by Rhee et al.,31 where all the patients experienced shock and a higher rate of resistance to antimicrobial agents. On the other hand, a study conducted in Taiwan, in 2009, with 33 patients reported that hypotension, renal function, and liver cirrhosis were associated with higher mortality rate. All isolates were susceptible to gentamicin, amikacin, ceftazidime, cefepime, and ciprofloxacin.29 Mortality associated with CAI was higher than with HAI, but there was not enough data to allow for a definitive conclusion.
Some studies reported mortality rates between 28% and 46% associated with Aeromonas bacteremia32; however, the incidence of sepsis was relatively low, less than 15% of cases.33 Our study found five cases with bacteremia, which could be related to the fact that most of these patients had higher severity indexes and greater use of invasive devices.
LimitationsOur study has limitations, and our descriptions should be interpreted in the context of the study design. First, we used commercial systems for Aeromonas identification in routine conditions, but we did not consider 16 s rRNA sequencing genetic test to differentiate each bacteria phylogenetically (reference standard). Second, only 11 Aeromonas spp. isolates were analyzed for PCR amplification as it was not possible to recover the isolates from skim milk medium. Third, due to the study design and sample size, selection bias could have been introduced. Despite these limitations, our description is a call for action to mitigate Aeromonas antibiotic resistance trends.
ConclusionsEmerging phenotypic carbapenem resistance infection has being reported in our hospital, mostly as HAI. There was a high mortality rate, especially in immunocompromised patients and in those who fail empirical treatment with carbapenems. As the main carbapenemase tests were negative, carbapenem resistance could be attributed to intrinsic MBLs, CphA encoded by the CphA gene, and/or possible hyperproduction of ampC β-lactamase and porins expression. Also, the EDTA test that searches for MBLs can be less accurate because there could be a reduced expression of CphA that generates false-negative results, this gene expression can change depending on the strain and the species.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the Centro de Investigaciones Clínicas (CIC) – Fundación Valle del Lili and Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) for their constant support.