The clinical significance of coexistence of HBsAg/anti-HBs in chronic hepatitis B (CHB) patients remains controversial. This study was aimed to assess the association of this serological pattern with hepatocellular carcinoma (HCC) in patients with CHB.
MethodsIn this cross-section study, 206 CHB patients with coexistence of HBsAg/anti-HBs and 206 CHB patients with HBsAg alone were included to evaluate the risk of HCC development by logistic regression analysis. In addition, a retrospective cohort of 260 patients with CHB was recruited to estimate the cumulative incidence of HCC by Kaplan–Meier analysis.
ResultsThe serological pattern of coexistence of HBsAg/anti-HBs, with high levels of (“High”) HBsAg/low levels of (“Low”) anti-HBs, were considered as independent risk factors for HCC. In particular, patients with “High” HBsAg/“High” anti-HBs [odds ratio (OR), 4.295; 95% confidence interval (CI), 1.104–16.699; p = 0.035] and “Low” HBsAg/ “High” anti-HBs (OR, 3.207; 95%CI, 1.299–7.919; p = 0.012) exhibited significantly higher risk for HCC development. However, only “Low” HBsAg /“High” anti-HBs might increase risk of HCC in CHB patients with high HBV load (logrank p < 0.001) in our cohort study.
ConclusionThe coexistence of “Low” HBsAg /“High” anti-HBs might increase the risk of HCC development in CHB patients with high HBV load, which reflected that the long-term interaction between immune response and virus might lead to the development of HCC. The identification of the patients with poor prognosis will help clinicians to refine the therapeutic decisions and individualize follow-up strategies.
Chronic hepatitis B virus (HBV) infection is widely spread throughout the world. According to “Global Hepatitis Report, 2017” by the World Health Organization, 257 million people or 3.5% of the global population were living with chronic HBV infection in 2015 worldwide. In addition, 20% or more of those with chronic infection developed end-stage chronic liver disease, such as cirrhosis or hepatocellular carcinoma (HCC). At present, routine laboratory tests to detect HBV infection are mainly serological and molecular assays. In recent years, some rare serological patterns appeared more frequently, including coexistence of HBsAg and anti-HBs, isolate positivity for HBsAg, and ‘anti-HBc alone.’1,2 The serological profile for coexistence of HBsAg and anti-HBs was firstly reported by Arnold et al.3–8 However, the underlying mechanism and clinical implication of this serological pattern in chronic hepatitis B patients remains controversial.9,10 Many reports have suggested that this coexistent serological pattern might increase the risk of HCC in chronic HBV infection.11,12 However, the coexistence of antigen and antibody is a matter stoichiometry; the clinical significance of this serological pattern, which were based on quantitation of HBsAg and anti-HBs, has not been definitively understood. The aim of the present study was to assess the association between coexisting of HBsAg/anti-HBs and development of HCC in CHB patients.
Material and methodsStudy subjects and designInitially, a cross-section study carried out from January 2017 to March 2018, involving 206 medical inpatients with CHB at Beijing You an Hospital, Capital Medical University, were enrolled as Group 1 (coexistence of HBsAg and anti-HBs) according to the inclusion and exclusion criterion (Table 1). As controls, 206 medical inpatients were randomly selected as Group 2 (HBsAg alone). Medical records of these 412 patients were reviewed, including clinical evaluations, imaging features and laboratory results. Demographic, clinical and serological characteristics of Group 1 and Group 2 were compared, and the risk of developing HCC was assessed through logistic regression analysis.
The eligibility criteria for the cross-sectional study.
| Group 1 | Group 2 |
|---|---|
| Inclusion | |
| 1. Age ≥18 years; | 1. Age ≥18 years; |
| 2. Concomitant HBsAg and anti-HBs positive for at least 6 months and without vaccination; | 2. HBsAg positive alone for at least 6 months; |
| Exclusion | |
| 1. Positive for HCV or HDV IgG; | |
| 2. Metastatic liver cancer; | |
| 3. After organ transplant; | |
| 4. On hemodialysis; | |
| 5. On immunosuppressive drugs; | |
| 6. With HIV infection; | |
| 7. Use of HBIG; | |
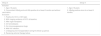
Furthermore, a cohort study with chronic HBV infection patients compared the development of HCC in Group 3 (130 patients with coexistence of HBsAg and anti-HBs) and Group 4 (130 patients with HBsAg alone). The cohorts were retrospectively collected between September 2010 and November 2013, according to the eligibility criteria listed in Table 2. The flow chart is presented in Fig. 1. The follow-up duration was calculated from the initial visit to the last visit or the diagnosis of HBV-associated HCC. The medical records all patients were reviewed, including clinical evaluations, imaging features and laboratory results. All patients were followed by ultrasonography (US) or liver dynamic three-dimensional computed tomography (CT) and serum alpha-fetoprotein every 6–12 months. During follow-up, that ranged from 9 to 92 months, HCC developed in 20 (15.4%) of Group 3 and in 10 (7.7%) patients of Group 4. Since early studies had confirmed an increasing risk of HCC when HBV DNA level was over 2000 IU/mL (high HBV load),13,14 the cohort was further divided into two subgroups (high HBV load and low HBV load). Cumulative incidence of HCC was then estimated using Kaplan–Meier analysis.
The eligibility criteria for the cohort study.
| Group 3 | Group 4 |
|---|---|
| Inclusion | |
| 1. Age ≥18 years; | 1. Age ≥18 years; |
| 2. Concomitant HBsAg and anti-HBs positive for at least 6 months and without vaccination; | 2. HBsAg positive alone for at least 6 months; |
| Exclusion | |
| 1. Positive for HCV or HDV IgG; | |
| 2. With imaging evidence of HCC at baseline; | |
| 3. After organ transplant; | |
| 4. On hemodialysis; | |
| 5. On immunosuppressive drugs; | |
| 6. With HIV infection; | |
| 7. Undergoing liver transplantation during the follow-up period; | |
| 8. Receiving antiviral therapy before. | |
Case definitions of chronic CHB and HBV-associated cirrhosis were in accordance with the criteria issued by the Association of Infectious Diseases of China in 2015.15 HBV-associated HCC was diagnosed according to standardization of diagnosis and treatment for primary liver cancer.16 The liver failure was diagnosed according to the guideline from the Association of Infectious Diseases of China in 201217 and categorized into four groups: acute liver failure (ALF), subacute liver failure (SALF), acute-on-chronic liver failure (ACLF), and chronic liver failure (CLF).
Clinical laboratory testsThe HBV serological markers HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc were detected under Roche Elecsys e601 (Roche, German). Anti-HBs with results over 10 mIU/mL were considered positive. Serum anti-HBs levels ≥100 mIU/mL were regarded as ‘High’ anti-HBs (high levels of anti-HBs), for individuals with anti-HBs ≥100 mIU/mL were recognized to have sufficient immunity against HBV infection. It was necessary to maintain anti-HBs ≥100 mIU/mL so as to prevent reinfection of HBV in liver transplant recipients by using HBV immunoglobulin (HBIG).18 Therefore, samples with positive anti-HBs and serum levels <100 mIU/mL were regarded as ‘Low’ anti-HBs (low levels of anti-HBs). It was reported that HBsAg ≥1000 IU/mL was a key factor associated with HCC development in patients with HBV DNA level <2000 IU/mL.13 Thus, serum levels ≥1000 IU/mL were regarded as ‘High’ HBsAg (high levels of HBsAg). Similarly, samples with positive HBsAg with serum levels <1000 IU/mL were regarded as ‘Low’ HBsAg (low levels of HBsAg).
Serum alanine aminotransferase (ALT) levels were determined under ADVIA 2400 (Siemens, Germany). Serum alpha-fetoprotein (AFP) levels were also determined by Roche Elecsys e601 (Roche, German), and serum levels induced by vitamin K absence or antagonist II (PIVKA-II) were detected by using the commercial kit (Fujirebio, Japan) of chemiluminescent microparticle immunoassay. Serum HBV DNA levels were quantified by COBAS Taqman HBV DNA (Roche, German) with a limit detection threshold of 20 IU/mL.
Statistical analysisThe Mann–Whitney U-test was used to compare age, ALT, AFP, PIVKA-II, and HBV DNA among groups, while the chi-squared test was used to compare categorical variables such as the proportions of age ranges, sex, the grade of Child–Pugh. Logistic regression analysis was performed using candidate variables based on univariate analysis. Kaplan–Meier analysis was performed to estimate the cumulative incidence of HCC in patients including Group 3 and Group 4, and the two cumulative incidences were compared with the log rank test. Data from medical records were stored using Microsoft Office Excel 2010 (Microsoft Corp.). The software of SPSS v. 24.0 (IBM Corp.) was used for data analysis. All p-values were two-sided and p-values <0.05 were considered statistically significant.
ResultsThe cross-section studyThe overall (n = 412) median age study subjects was 52 years (range, 19–91), 76.5% (315/412) male; 44.4% (183/412) were diagnosed with HCC, 10.4% (43/412) had evidence of liver failure, 18.4% (76/412) were evaluated as Child–Pugh C.
Characteristics of patients with coexistence of HBsAg and anti-HBsDemographic, clinical and serological characteristics of the study groups are summarized in Table 3. In brief, there were no significant differences between Group 1 and 2 regarding sex (p = 0.131), grade of Child–Pugh (p = 0.488), presence of liver failure (p = 0.903), serum ALT level (p = 0.231), HBeAg positive rate (p = 0.767) and serum levels of HBV DNA (p = 0.727). However, the median age of patients in Group 1 was significantly higher than in Group 2 (54 years vs. 49 years; p < 0.001). It should be pointed out that there was a higher proportion of patients with HCC and also a higher proportion of patients with liver failure in Group 1 than in Group 2 (51.0% vs. 37.8%, p = 0.011; 13.6% vs. 7.3%, p = 0.036, respectively). The proportion of patients with HBsAg ≥1000 IU/mL in Group 1 was significantly higher than Group 2 (38.3% vs. 54.9%, p = 0.001). Moreover, the median serum AFP level of the patients was 32.7 (P25–P75, 6.2–296.2) ng/mL in Group 1 and 16.4 (P25–P75, 4.9–156.5) U/L in Group 2 (p = 0.024). Similarly, the median serum PIVKA-II level of patients in Group 1 was higher than in Group 2 (p < 0.001). Interestingly, the coexistence of HBeAg and anti-HBe was more frequently found in patients with coexistence of HBsAg and anti-HBs than patients in Group 2 (39.8% vs. 22.1%, p = 0.008).
Demographic, clinical and laboratory data of patients with coexistence of HBsAg and anti-HBs (Group 1) and HBsAg alone (Group 2).
| Group1(n = 206) | Group 2(n = 206) | p-value | |
|---|---|---|---|
| Age (years)a | 54 (4562) | 49 (3956) | <0.001 |
| Male sex, n (%) | 151 (73.3) | 164 (79.6) | 0.131 |
| Classification of liver disease, n | 0.011 | ||
| CHB, n (%) | 39 (18.9) | 50 (24.8) | |
| Cirrhosis, n (%) | 62 (30.1) | 78 (37.4) | |
| HCC, n (%) | 105 (51.0) | 78 (37.8) | |
| Child–Pugh, n | 0.488 | ||
| A, n (%) | 99 (48.1) | 108 (52.4) | |
| B, n (%) | 67 (32.5) | 62 (30.1) | |
| C, n (%) | 40 (19.4) | 36 (17.5) | |
| Liver failure, n (%) | 28 (13.6) | 15 (7.3) | 0.036 |
| Classification of liver failure, n | 28 | 15 | 0.903 |
| ALF, n (%) | 0 (0) | 0 (0) | |
| SALF, n (%) | 1 (3.6) | 1 (6.7) | |
| ACLF, n (%) | 23 (82.2) | 12 (80.0) | |
| CLF, n (%) | 4 (14.2) | 2 (13.3) | |
| Laboratory results | |||
| ALT (IU/L)a | 66.0 (31.0, 162.4) | 51.9 (26.3, 245.0) | 0.231 |
| HBsAg ≥1000 IU/mL, n (%) | 79 (38.3) | 113 (54.9) | 0.001 |
| HBeAg positive, n (%) | 98 (47.6) | 95 (46.1) | 0.767 |
| HBeAg/anti-HBe co-positive, n (%)b | 39 (39.8) | 21 (22.1) | 0.008 |
| AFP (ng/ml)a | 32.7 (6.2, 296.2) | 16.4 (4.9, 156.5) | 0.024 |
| PIVKA-II (mAU/mL)a | 45 (24,980) | 30 (1980) | <0.001 |
| HBV-DNAa,c | 4.7 (2.5, 6.1) | 4.0 (2.0, 6.6) | 0.727 |
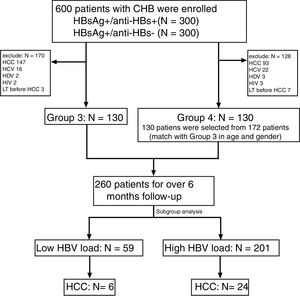
Four serological patterns of different serum levels combinations of HBsAg and anti-HBs are exhibited in Fig. 2. Out of the 206 patients with coexistence of HBsAg and anti-HBs, the proportion of ‘Low’ HBsAg / ‘Low’ anti-HBs was the highest (40.29%), while the number of patients with ‘High’ HBsAg / ‘High’ anti-HBs was the lowest (9.71%). The rate of HCC were higher in Group 1 than in Group 2. However, there was no significant difference in the proportions of HCC patients within these different serological patterns (P = 0.827).
The proportion of different serological patterns of HBsAg and anti-HBs in the cross-sectional study. H/H: ‘High’ HBsAg / ‘High’ anti-HBs. H/L: ‘High’ HBsAg / ‘Low’ anti-HBs. L/H: ‘Low’ HBsAg / ‘High’ anti-HBs. L/L: ‘Low’ HBsAg / ‘Low’ anti-HBs. +/−: HBsAg positive and anti-HBs negative. CHB: chronic hepatitis B. HCC: hepatocellular carcinoma. The proportions of HCC in patients with all four serological patterns of coexistence of HBsAg and anti-HBs are higher than patients with HBsAg positive and anti-HBs negative. However, there are no obvious differences in the proportions of HCC in patients with these different serological patterns (p = 0.827).
In univariate analysis, age ≥50 years, male sex, Child–Pugh C, liver failure, initial serum ALT level, coexistence of HBsAg and anti-HBs, HBeAg positive, and serum HBV DNA level were significantly associated with HCC (Table 4).
Univariate analysis of risk factors for HCC (Combined patients of Group 1 and Group 2).
| HCC (n = 183) | Non-HCC (n = 229) | χ2/U | p-value | ||
|---|---|---|---|---|---|
| Age | 52.870 | <0.001 | |||
| <50 years, n (%) | 44 (24.0) | 137 (59.8) | |||
| ≥50 years, n (%) | 139 (76.0) | 92 (40.2) | |||
| Sex | 5.555 | 0.018 | |||
| Male, n (%) | 150 (82.0) | 165 (72.1) | |||
| Female, n (%) | 33 (18.0) | 64 (27.9) | |||
| Child–Pugh C | 9.034 | 0.003 | |||
| Yes, n (%) | 22 (12.0) | 54 (23.6) | |||
| No, n (%) | 161 (88.0) | 175 (76.4) | |||
| Liver failure | 22.157 | <0.001 | |||
| Yes, n (%) | 4 (2.2) | 37 (16.2) | |||
| No, n (%) | 179 (97.8) | 192 (83.8) | |||
| ALT(IU/L)a | 37.5 (23.5, 71.8) | 128.7 (38.5, 641.2) | 11,186 | <0.001 | |
| Coexistence of HBsAg/anti-HBs | 7.167 | 0.007 | |||
| Yes, n (%) | 105 (57.4) | 101 (44.4) | |||
| No, n (%) | 78 (42.6) | 128 (55.6) | |||
| HBeAg | 17.893 | <0.001 | |||
| Positive, n (%) | 64 (35.0) | 128 (55.9) | |||
| Negative, n (%) | 119 (65.0) | 101 (44.1) | |||
| HBeAg positive | 0.306 | 0.580 | |||
| Anti-HBe positive, n (%) | 18 (28.1) | 41 (32.0) | |||
| Anti-HBe negative, n (%) | 46 (71.9) | 87 (68.0) | |||
| HBV-DNAa,b | 3.2 (2.0, 4.9) | 5.5 (2.7, 7.1) | 30,225 | <0.001 | |
In multivariate logistic regression analysis, age ≥50 years [odds ratio (OR), 4.486; 95% confidence interval (CI), 2.592–7.762; p < 0.001], male sex (OR, 3.683; 95% CI, 2.025–6.699; p < 0.001), and coexistence of HBsAg and anti-HBs (OR, 2.063; 95% CI, 1.259–3.382; p = 0.004) were considered as independent risk factors for HCC (Table 5). The different serological patterns for coexistence of HBsAg and anti-HBs, except for ‘High’ HBsAg and ‘Low’ anti-HBs, were also considered as independent risk factors for HCC. In particular, compared to patients with HBsAg positive and anti-HBs negative, patients with the ‘High’ HBsAg /‘High’ anti-HBs (OR, 4.295; 95% CI, 1.104–16.699; p = 0.035) and ‘Low’ HBsAg / ‘High’ anti-HBs (OR, 3.207; 95% CI, 1.299–7.919; p = 0.012) exhibited significantly higher risk for HCC (Table 5).
The multivariate logistic regression analysis of risk factors for HCC (Combined patients of Group 1 and Group 2).
| Variables | Frequency | OR | 95%CI | p-value |
|---|---|---|---|---|
| Age ≥50 years | ||||
| No | 181 | 1 | ||
| Yes | 231 | 4.486 | 2.592–7.762 | <0.001 |
| Sex | ||||
| Female | 97 | 1 | ||
| Male | 315 | 3.683 | 2.025–6.699 | <0.001 |
| Child–Pugh C | ||||
| No | 336 | 1 | ||
| Yes | 76 | 0.715 | 0.333–1.533 | 0.389 |
| Liver failure | ||||
| No | 369 | 1 | ||
| Yes | 43 | 0.122 | 0.035–0.429 | <0.001 |
| ALT(IU/L)a | 58.9 (28.5, 184.9) | 0.999 | 0.998–1.000 | 0.023 |
| HBsAg+/anti-HBs+ | ||||
| No | 206 | 1 | ||
| Yes | 206 | 2.063 | 1.259–3.382 | 0.004 |
| HBsAg+/anti-HBs+ | ||||
| No | 206 | 1 | ||
| L/L | 83 | 2.517 | 1.190–5.324 | 0.016 |
| L/H | 44 | 3.207 | 1.299–7.919 | 0.012 |
| H/L | 59 | 1.812 | 0.821–3.997 | 0.141 |
| H/H | 20 | 4.295 | 1.104–16.699 | 0.035 |
| HBeAg positive | ||||
| No | 220 | 1 | ||
| Yes | 192 | 1.047 | 0.585–1.873 | 0.877 |
| HBV-DNAa,b | 4.2 (2.2–6.2) | 0.752 | 0.654–0.865 | <0.001 |
The baseline characteristics of the study sample are presented in Table 6. Lower median serum HBV DNA levels and shorter median follow-up duration in Group 3 than Group 4 (5.3 log10 IU/mL vs. 6.0 log10 IU/mL, p = 0.041; 54.5 months vs. 68.5 months, p = 0.041, respectively) were found. There were no significant differences between these two groups, in terms of age (p = 0.567), male sex (p = 0.274), proportion of Child–Pugh C (p = 0.169), serum ALT (p = 0.186), proportion of cirrhosis (p = 0.380), and rate of patients with HBeAg positive.
Baseline characteristics of patients in Group 3 and Group 4.
| Group 3(n = 130) | Group 4 (n = 130) | χ2/U | p-value | |
|---|---|---|---|---|
| Age(years)a | 47 (3654) | 43 (3453) | 8103.5 | 0.567 |
| Male Sex, n (%) | 101 (77.7) | 108 (83.1) | 1.195 | 0.274 |
| Child–Pugh C, n (%) | 16 (12.3) | 24 (18.5) | 1.891 | 0.169 |
| ALT(IU/L)a | 175.3 (46.4, 753.0) | 109.6 (46.9, 505.7) | 7648.5 | 0.186 |
| Cirrhosis, n (%) | 59 (45.4) | 52 (40.0) | 0.770 | 0.380 |
| HBeAg Positive, n (%) | 78 (60.0) | 87 (66.9) | 1.344 | 0.246 |
| HBV-DNAa,b | 5.3 (3.6, 6.6) | 6.0 (3.8, 7.4) | 7214 | 0.041 |
| Follow-up duration(months)a | 54.5 (33.0, 74.3) | 68.5 (33.8, 80.3) | 7208.5 | 0.041 |
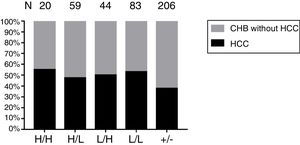
The incidence of HCC during the follow-up period was 15.4% (20/110) in patients with coexistence of HBsAg and anti-HBs and 7.7% (10/120) in those with HBsAg alone. Out of the 260 patients, 223 (85.8%) received antiviral therapy, and there was no significant difference in the proportion of patients who received antiviral therapy between the two groups (p = 0.214). The cumulative incidence of HCC was not related to coexistence of HBsAg and anti-HBs in a sub-cohort of 59 patients with HBV-DNA level <2000 IU/mL (p = 0.902) (Fig. 3A). However, in a subcohort of 201 patients with HBV-DNA level ≥2000 IU/mL, the cumulative incidence of HCC was associated with this serological pattern (p = 0.018) (Fig. 3B). Moreover, the cumulative incidence of HCC was also associated with different serological patterns of HBsAg and anti-HBs in patients with high HBV load (p < 0.001) (Fig. 3C). Particularly, ‘Low’ HBsAg / ‘High’ anti-HBs might be associated with higher risk of HCC development. To validate this finding, patients with HBV DNA level ≥2000 IU/mL were categorized by the different serological pattern: ‘Low’ HBsAg/‘Low’ anti-HBs (Fig. 3D), ‘High’ HBsAg/‘Low’ anti-HBs (Fig. 3E) and ‘Low’ HBsAg/‘High’ anti-HBs (Fig. 3F). Only the serological pattern of ‘Low’ HBsAg/‘High’ anti-HBs was associated with increased risk of HCC in patients with high HBV load (log rank p < 0.001). Unfortunately, only four patients with ‘High’ HBsAg and ‘High’ anti-HBs were included in our cohort study, and none of them developed HCC at the end of follow-up.
The cumulative incidence of HCC in patients with CHB. (A) The cumulative incidence of HCC is not related to coexistence of HBsAg and anti-HBs in a subcohort of 59 patients with HBV-DNA level <2000 IU/mL (p = 0.902). (B) However, in a subcohort of 201 patients with HBV-DNA level ≥2000 IU/mL, the cumulative incidence of HCC is associated with coexistence of HBsAg and anti-HBs (P = 0.018). (C) Comparison of cumulative incidence of HCC in different serological patterns of HBsAg/anti-HBs. These patients with HBV DNA level ≥2000 IU/mL are categorized by the different serological pattern: ‘Low’ HBsAg / ‘Low’ anti-HBs (D), ‘High’ HBsAg / ‘Low’ anti-HBs (E), and ‘Low’ HBsAg / ‘High’ anti-HBs (F).
The prevalence of HBsAg and anti-HBs co-positive patterns in chronic HBV-infected patients ranged from 4.9% (20/411) in a Chinese cohort to 8.9% (77/864) in a French cohort.19,20 The prevalence rate in Korea was reported as 7.0% (73/1042).12 Obviously, this serological pattern is ubiquitous, and its clinical significance is worth to be explored. As HCC is a devastating complication for CHB patients, it is important to identify the risk factors for this complication in clinical practice.21 Although it has been reported that coexistence of HBsAg and anti-HBs might increase the risk of HCC in CHB infection,11,12 the potential association of different serological patterns of quantitative HBsAg and anti-HBs with HCC had not been investigated before. We attempted to find out whether the different serological pattern of coexistence of HBsAg and anti-HBs was associated with increased risk of HCC in CHB patients.
In the cross-sectional study, some serological patterns of coexistence of HBsAg and anti-HBs (‘High’ HBsAg/‘High’ anti-HBs, ‘Low’ HBsAg/‘High’ anti-HBs, and ‘Low’ HBsAg/‘Low’ anti-HBs) were considered as independent risk factors for HCC. Particularly, by comparing to patients with HBsAg alone, patients with the ‘High’ HBsAg / ‘High’ anti-HBs and ‘Low’ HBsAg / ‘High’ anti-HBs exhibited significantly higher risk for HCC. In the cohort study, survival analysis was used to identify the causal association by a long-term follow-up cohort. However, high HBV load (HBV DNA ≥2000 IU/mL) in CHB patients suggested active viral replication, which was considered as the driving force of disease progression.22,23 Patients with low HBV load had minimal risk of HCC originally. It might explain the reason why coexistence of HBsAg and anti-HBs did not increase risk of HCC, compared to HBsAg alone in CHB patients with low HBV load. Overall, presence of anti-HBs and HBsAg suggested an ongoing immune response which was insufficient to control the chronic HBV infection. The continuous interaction of immune response and viral replication led to chronic inflammation, which is an acknowledged mechanism contributing to the development of HCC. Therefore, in our study, coexistence of HBsAg and anti-HBs was a factor associated with HCC development in CHB patients with high HBV load. However, in patients with high HBV load, only the serological pattern of ‘Low’ HBsAg/‘High’ anti-HBs, but not ‘High’ HBsAg/ ‘High’ anti-HBs, was associated with increased risk of HCC, which was mainly attributed to the hypothesis discussed below. High HBV load indicates active viral replication, while high levels of anti-HBs blocked infected hepatocytes from releasing wild-type HBsAg.24 Meanwhile, a strong immune stress exerted by high levels of anti-HBs induced the selection and emergence of HBV variants with mutations in the preS/S genomic region. Therefore, the accumulation of envelope proteins, particularly preS mutations, in endoplasmic reticulum of infected hepatocytes might activate the endoplasmic stress-signaling pathways, which induce oxidative DNA damage and genomic instability. Therefore, the cytotoxic effects could lead to liver damage, favoring the development of HCC.25 In contrast, the attribution of the serum levels of HBsAg to active host’s immune system in spite of intracellular high expression of HBsAg might be low. Thus, this serological pattern could be related to HCC development.
There are several limitations in our research. Firstly, all research subjects selected were from our institute in northern China. It has been confirmed that HBV infection in northern areas is mainly HBV genotype C, which has a prolonged active viral DNA replication and protein expression, thus increasing the lifelong risk for liver cirrhosis and HCC.26 Secondly, since all participants in the cross-sectional study were medical inpatients, it may have introduced selection bias by including a higher proportion of HCC patients than in the general population. Thirdly, the association of serological pattern with HCC could not be observed in the long run due to insufficient follow-up. At last, in the cohort study, the number of patients with ‘High’/‘High’ HBsAg and anti-HBs was not big enough for further analysis of the relation between serological pattern and HCC development.
In conclusion, coexistence of HBsAg and anti-HBs, particularly for ‘Low’ HBsAg and ‘High’ anti-HBs, might be associated with an increased risk of HCC development in CHB patients with high HBV load. The interesting results may be attributed to the active chronic inflammation induced by the long-term interaction between host’s immune response and virus. Therefore, the emergence of high antibodies levels may lead clinicians to make a false judgment of disease recovery. In clinical practice, ‘Low’ HBsAg/‘High’ anti-HBs in CHB patients with high HBV load should take the possibility of risk for the HBV-related adverse outcomes into consideration. If this pattern is detected in patients with CHB, it is recommended to check for HBV DNA and HBV mutation and to alert against the occurrence of HCC-associated mutants, regardless of receiving antiviral therapy or not. The identification of patients with poor prognosis will help clinicians to refine the therapeutic interventions and to individualize follow-up strategies to prevent or detect HCC early.
Declarations of interestNone.
Funding sourcesThis study was supported by the National Science and Technology Major Project (grant no. 2018ZX10305-410-006) and the 2018 Beijing Youan Hospital Scientific Research Project for Young & Middle-Aged Talent’s Cultivation (grant no. YNKTTS20180104) to Jinli Lou.
Ethical approvalThis research was approved by the Ethics Committee of Beijing You an Hospital, Beijing, China