Colistin resistance involving Gram-negative bacilli infections is a challenge for health institutions around of the world. Carbapenem-resistance among these isolates makes colistin the last therapeutic option for this treatment. Colistin resistance among Enterobacteriaceae, Acinetobacter spp., and Pseudomonas spp. was evaluated between 2010 and 2014 years, at Hospital das Clínicas, São Paulo, Brazil. Over five years 1346 (4.0%) colistin resistant Gram-negative bacilli were evaluated. Enterobacteriaceae was the most frequent (86.1%) pathogen isolated, followed by Acinetobacter spp. (7.6%), and Pseudomonas spp. (6.3%). By temporal analysis there was a trend for an increase of colistin resistance among Enterobacteriaceae, but not among non-fermentative isolates. Among 1346 colistin resistant isolates, carbapenem susceptibility was observed in 21.5%. Colistin resistance in our hospital has been alarmingly increased among Klebsiella pneumoniae isolates in both KPC positive and negative, thus becoming a therapeutic problem.
The dissemination of infections caused by multi-drug resistant Gram-negative isolates has become a major public heath challenge worldwide. According to the last report of the Brazilian Health Surveillance Agency (ANVISA), Klebsiella pneumoniae is the third pathogen isolated from patients admitted to intensive care units – ICU (13.8%), followed by Acinetobacter spp. (11.8%), and Pseudomonas spp. (10.1%).1 These pathogens exhibited elevated rates of resistance to carbapanems, mainly among Acinetobacter spp. isolates (80.7%).1 In this circumstances, polymyxins are one of the last therapeutic options; however, reports of resistance to colistin around the world are increasing over time.2–4
The carbapenem resistance rates among Acinetobacter spp. isolates in our institution, increased from 30% to 70%, between 2010 and 2014 (data not showed). Therefore, colistin became the first-line drug for Gram-negative infections in patients admitted to ICU in the last five years. Few data on colistin resistance in Brazil are currently found in the literature. Despite the good activity still displayed by polymyxins against non-fermentative microorganisms, reduction in susceptibility rates has been observed among Enterobacteriaceae.5,6 In this study, colistin resistance among Enterobacteriaceae, Acinetobacter spp., and Pseudomonas spp. clinical isolates was retrospectively evaluated from the database of Microbiology Laboratory of Hospital das Clínicas, São Paulo, Brazil, between 2010 and 2014. No surveillance or community-acquired isolates were included in the analysis, and only one isolate for each patient was evaluated in the study. The clinical samples were received from nine hospitals of São Paulo city: Hospital das Clínicas – Instituto Central; Instituto do Câncer do Estado de São Paulo – ICESP; Instituto do Coração – INCOR; Instituto de Ortopedia e Trauma – IOT; Instituto de Psiquiatria – IPQ; Instituto de Radiologia – INRAD; Hospital do Cotoxó; Instituto da Criança – ICR, and Hospital de Suzano. The data were analyzed using Excel and WHONET 5.6 softwares (World Health Organization, http://www.whonet.org/www/software.html).
The identification of isolates and colistin susceptibility test were performed by Vitek 2 (bioMérieux, France), and the colistin-resistant MICs were confirmed by Etest methods, according to CLSI recommendations.7 The Etest method was previously validated on the microbiology laboratory using broth microdilution (Trek Diagnostics Systems), according to CLSI recommendations.7 The susceptibility categories were interpreted according to break-points of CLSI guidelines8 for non-fermentative isolates and EUCAST9 for Enterobacteriaceae members.
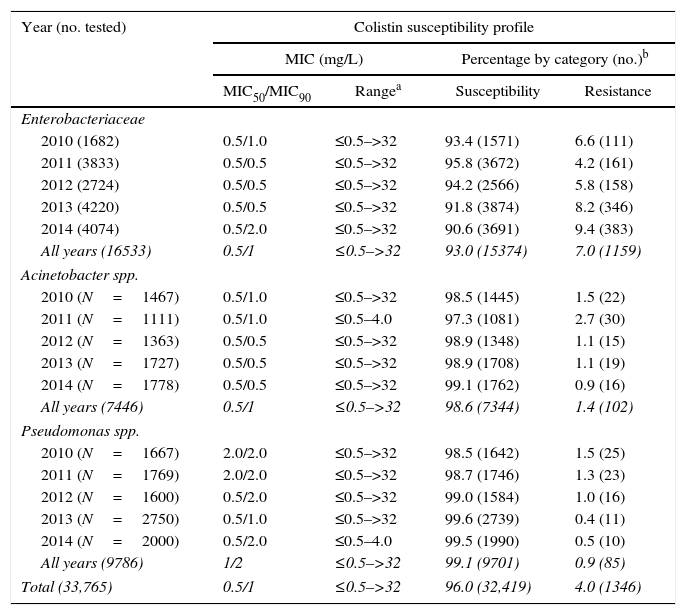
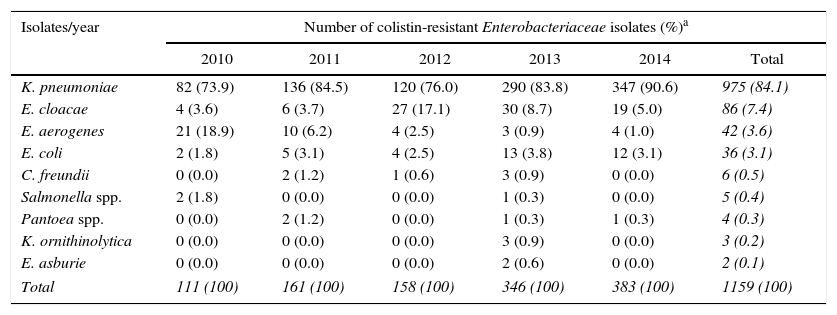
Between 2010 and 2014, 33,765 Gram-negative bacilli from non-repeated patients were tested for colistin resistance in the microbiology laboratory. Enterobacteriacea species was the most frequent group representing 49% (16,533 isolates) of all Gram-negative tested, followed by Pseudomonas spp. and Acinetobacter spp. (29% [9786 isolates] and 22% [7446 isolates], respectively). Four percent (1346 isolates) of all Gram-negative bacilli tested were resistant to colistin with MICs ranging from 2 to >32mg/L. Among colistin-resistant isolates, Enterobacteriaceae were recovered most frequently from urine (34.8%), while Acinetobacter spp. and Pseudomonas spp. were more often isolated from respiratory samples (45% and 35.3%, respectively). Seven percent (1159 isolates) of Enterobacteriaceae isolates were resistant to colistin, while 1.4% (102 isolates) of Acinetobacter spp. and 0.9% (85 isolates) of Pseudomonas spp. showed and colistin resistance over the five years evaluated in this study (Table 1). Among Enterobacteriaceae members, K. pneumoniae was the most frequent with 975 isolates (84.1%), followed by Enterobacter cloacae (86 isolates, 7.4%), E. aerogenes (42 isolates, 3.6%), Escherichia coli (36 isolates, 3.1%), Citrobacter freundii (6 isolates, 0.5%), Salmonella spp. (5 isolates, 0.4%), Pantoea spp. (4 isolates, 0.3%), K. ornithinolytica (3 isolates, 0.2%), and E. asburiae (2 isolates, 0.1%) (Table 2).
Susceptibility and resistance rates among Enterobacteriaceae, Acinetobacter spp., and Pseudomonas spp. isolated to colistin between 2010 and 2014, in HC-FMUSP.
| Year (no. tested) | Colistin susceptibility profile | |||
|---|---|---|---|---|
| MIC (mg/L) | Percentage by category (no.)b | |||
| MIC50/MIC90 | Rangea | Susceptibility | Resistance | |
| Enterobacteriaceae | ||||
| 2010 (1682) | 0.5/1.0 | ≤0.5–>32 | 93.4 (1571) | 6.6 (111) |
| 2011 (3833) | 0.5/0.5 | ≤0.5–>32 | 95.8 (3672) | 4.2 (161) |
| 2012 (2724) | 0.5/0.5 | ≤0.5–>32 | 94.2 (2566) | 5.8 (158) |
| 2013 (4220) | 0.5/0.5 | ≤0.5–>32 | 91.8 (3874) | 8.2 (346) |
| 2014 (4074) | 0.5/2.0 | ≤0.5–>32 | 90.6 (3691) | 9.4 (383) |
| All years (16533) | 0.5/1 | ≤0.5–>32 | 93.0 (15374) | 7.0 (1159) |
| Acinetobacter spp. | ||||
| 2010 (N=1467) | 0.5/1.0 | ≤0.5–>32 | 98.5 (1445) | 1.5 (22) |
| 2011 (N=1111) | 0.5/1.0 | ≤0.5–4.0 | 97.3 (1081) | 2.7 (30) |
| 2012 (N=1363) | 0.5/0.5 | ≤0.5–>32 | 98.9 (1348) | 1.1 (15) |
| 2013 (N=1727) | 0.5/0.5 | ≤0.5–>32 | 98.9 (1708) | 1.1 (19) |
| 2014 (N=1778) | 0.5/0.5 | ≤0.5–>32 | 99.1 (1762) | 0.9 (16) |
| All years (7446) | 0.5/1 | ≤0.5–>32 | 98.6 (7344) | 1.4 (102) |
| Pseudomonas spp. | ||||
| 2010 (N=1667) | 2.0/2.0 | ≤0.5–>32 | 98.5 (1642) | 1.5 (25) |
| 2011 (N=1769) | 2.0/2.0 | ≤0.5–>32 | 98.7 (1746) | 1.3 (23) |
| 2012 (N=1600) | 0.5/2.0 | ≤0.5–>32 | 99.0 (1584) | 1.0 (16) |
| 2013 (N=2750) | 0.5/1.0 | ≤0.5–>32 | 99.6 (2739) | 0.4 (11) |
| 2014 (N=2000) | 0.5/2.0 | ≤0.5–4.0 | 99.5 (1990) | 0.5 (10) |
| All years (9786) | 1/2 | ≤0.5–>32 | 99.1 (9701) | 0.9 (85) |
| Total (33,765) | 0.5/1 | ≤0.5–>32 | 96.0 (32,419) | 4.0 (1346) |
Number of colistin-resistant Enterobacteriaceae family members isolated between 2010 and 2014, in HC-FMUSP.
| Isolates/year | Number of colistin-resistant Enterobacteriaceae isolates (%)a | |||||
|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | Total | |
| K. pneumoniae | 82 (73.9) | 136 (84.5) | 120 (76.0) | 290 (83.8) | 347 (90.6) | 975 (84.1) |
| E. cloacae | 4 (3.6) | 6 (3.7) | 27 (17.1) | 30 (8.7) | 19 (5.0) | 86 (7.4) |
| E. aerogenes | 21 (18.9) | 10 (6.2) | 4 (2.5) | 3 (0.9) | 4 (1.0) | 42 (3.6) |
| E. coli | 2 (1.8) | 5 (3.1) | 4 (2.5) | 13 (3.8) | 12 (3.1) | 36 (3.1) |
| C. freundii | 0 (0.0) | 2 (1.2) | 1 (0.6) | 3 (0.9) | 0 (0.0) | 6 (0.5) |
| Salmonella spp. | 2 (1.8) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 5 (0.4) |
| Pantoea spp. | 0 (0.0) | 2 (1.2) | 0 (0.0) | 1 (0.3) | 1 (0.3) | 4 (0.3) |
| K. ornithinolytica | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.9) | 0 (0.0) | 3 (0.2) |
| E. asburie | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.6) | 0 (0.0) | 2 (0.1) |
| Total | 111 (100) | 161 (100) | 158 (100) | 346 (100) | 383 (100) | 1159 (100) |
Since resistance to carbapenem in our institution is elevated, in the last five years colistin became the first-line drug for treating infections caused by Gram-negative pathogens in patients admitted to the ICUs of HC-FMUSP. Previous studies indicate that the emergence of colistin-heteroresistant populations may be amplified by colistin exposure.10,11 Data of Sentry Surveillance Program show high susceptibility rates to colistin worldwide until 2010 (Pseudomonas spp., 99.4%; Acinetobacter spp., 98.3%; and K. pneumoniae, 96.8%).3,12 The decrease of susceptibility to colistin among these isolates is a therapeutic challenge due to restricted therapeutic options. In our study, a trend of increase in colistin resistance rate was observed among Enterobacteriaceae family members, from 6.6% (111 isolates) in 2010 to 9.4% (383 isolates) in 2014 (Table 1). Since the first description of KPC-2-producing K. pneumoniae in Brazil, in 2009, studies have been published showing the spread of this carbapanemase, including to other bacterial species, in all regions.13–15 Over 80% of carbapenem-resistant K. pneumoniae from our institution is KPC-producer (data not showed). The spread of these phenotype and consequent increased use of colistin, may have contributed to the rise of colistin resistance rates observed in this study.
Fortunately, this trend was not observed among the non-fermentative isolates Acinetobacter spp. and Pseudomonas spp. In contrast, there was a slight reduction of colistin resistance rates (1.5% [22 isolates] to 0.9% [16 isolates] and 1.5% [25 isolates] to 0.5% [10 isolates], respectively), between 2010 and 2014 (Table 1). Previous Brazilian studies with carbapenem resistant P. aeruginosa and A. baumannii showed high susceptibility rates to colistin.7,16,17 In contrast, K. pneumoniae has demonstrated reduction of colistin susceptibility rates.18 Although resistance to colistin among Acinetobacter spp. in our Institution turned out to be not as high as compared to K. pneumoniae, it still represents a serious problem in our hospital, since carbapenem resistance among this species is elevated, and colistin is the only available antibiotic for treating these infections.
Furthermore, it should be pointed out that among colistin-resistant isolates 21.5% were susceptible to carbapenem (244 Enterobacteriaceae members [21.1%]; 23 Acinetobacter spp. [22.5%], and 23 Pseudomonas spp. [27.1%]). This phenotype is not frequently described, probably because many clinical laboratories only evaluate colistin susceptibility among carbapenem-resistant isolates. This phenotype deserves special attention because these isolates are also candidates to acquire resistance to carbapenems, leaving no therapeutic option for treating these infections. Olaitan et al.19 affirm that clinicians and microbiologists should be vigilant for the possible existence of colistin-resistant bacteria not only in patients undergoing colistin therapy, since such resistant bacteria could later be selected if colistin is used.
The main limitation of our study was the use of an automated method for determination of MIC to colistin. So, the Etest method was used to confirm the colistin MICs and reduce the possibility of errors. In addition, the Etest method was previously validated, comparing with broth microdilution results, according to CLSI recommendations. In addition, different brands of Mueller Hinton agar were evaluated during the Etest validation to avoid the possibility of misinterpretation due to influences of cations concentrations.20
In conclusion, in our institution colistin resistance is increasing among K. pneumoniae isolates, posing a real problem for treating these infections. Fortunately, this trend has not been observed among non-fermentative isolates that still remain highly susceptible to colistin. The increase of resistance to colistin, observed in this study, is a global reality and measures should be undertaken to prevent the complete loss of activity of this drug and dissemination of multi-drug resistant bacteria.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Rosilaine Souza Arruda Teberges by assistance on the WHONET data analysis. We also thank the Microbiology Laboratory group of HC-FMUSP for the great contribution to clinical routine.
This study was partially presented at 55th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Diego, EUA, 2015.