This study aimed to evaluate the clinical effectiveness in terms of sustained virological response and tolerability of available second generation direct-acting antivirals in Brazilian patients.
MethodsThis was a retrospective observational study conducted in six centers in Southern Brazil. The sample comprised adult patients who were chronically infected with hepatitis C virus, regardless of virus genotype, fibrosis stage, or prior treatment. Statistical analysis was performed to compare the effectiveness among the treatments, and also to uncover the factors influencing the achievement of sustained virological response.
ResultsA total of 296 patients were included in the study, with the majority receiving sofosbuvir with daclatasvir (59%) or sofosbuvir with simeprevir (26%). Overall sustained virological response rates were approximately 91.6%. For genotype 1, sofosbuvir with daclatasvir had an sustained virological response rate of approximately 95%, while the sustained virological response rate of sofosbuvir with simeprevir was 92%; this difference was statistically significant only for subtype 1b. The only treatment used for genotype 3 patients was sofosbuvir with daclatasvir, and lower rates of sustained virological response were observed for this group, compared to genotype 1 (84% versus 95%, p<0.05). Apart from this difference between genotypes, and a difference between patients who achieved rapid virologic response compared with those who did not, there were no other statistically significant factors associated with sustained virological response.
ConclusionsThe results point to the effectiveness of second-generation direct-acting antivirals in hepatitis C virus Brazilian patients, especially those with genotype 1. Furthermore, that patients with genotype 3 need more attention and adjustments in available treatment options.
Chronic hepatitis C is a serious health-related problem that affects more than 71 million people, causing almost 400,000 deaths worldwide every year.1 In Brazil, it is estimated that 1.5–1.7 million patients are infected with the hepatitis C virus (HCV), which represents about 2% of the population. However, a higher proportion of cases is from the South of Brazil (about 24.2% of the total number of cases in the country), where Curitiba city, the capital of Paraná state, is one of cities with the highest number of cases per 100,000 inhabitants (36.3).2–4 For many years, interferon-based therapies were the primary treatment choice for chronic hepatitis C, followed by the first generation of direct-acting antivirals (DAAs), boceprevir and telapravir, which were used in combination with pegylated interferon and ribavirin (RBV). However, these treatments were problematic due to limited efficacy, severe side effects, and contraindications.5,6
In the last few years, second generation DAAs have been developed to provide more effective, tolerable, and safe treatments for hepatitis C. These can be used in combination with other second generation DAAs, and without interferon.7–11 Since 2015 three DAA options have been available in the Brazilian public health system: sofosbuvir, daclatasvir, and simeprevir. The Brazilian government makes these drugs available at no cost, according to national guidelines for treating chronic hepatitis C, which prioritize patients with advanced stage of the disease. Recently, another combination of drugs was approved for use in Brazil, i.e., ombitasvir with paritaprevir/ritonavir and dasabuvir.4
These combinations of drugs have shown high rates of sustained virological response (SVR; a primary efficacy outcome measured at least 12 weeks after the end of treatment) and a favorable tolerability profile in randomized clinical trials (RCTs) and previous systematic reviews.12–14 It is expected that these outcomes would also be reproduced in clinical practice.
Observational and regional studies are needed for evidence to support decisions in clinical practice. It is well established that this type of study is an important step in investigating clinical outcomes for chronic diseases, especially because, in some situations, results from RCTs are not fully representative of the general population.15,16 Hepatitis C is a disease which is associated with many factors that can complicate medical treatment. This includes HCV genotype, co-infections with other viruses (such as the human immunodeficiency virus, HIV), and patient conditions (e.g., cirrhosis, liver transplantation, renal failure). Geographical differences in the populations studied can also affect treatment response due to varied viral characteristics of patients; this may occur in Brazil, which is a country with considerable extension and regional diversities.
In this context, the study aimed to evaluate the clinical effectiveness, in terms of SVR, and tolerability of second generation DAAs in chronic hepatitis C patients through an observational cohort study conducted in a Southern state of Brazil. We also aimed to compare the results obtained from patients receiving sofosbuvir with daclatasvir versus sofosbuvir with simeprevir.
Materials and methodsStudy design, eligibility criteria, and treatment outcomesIn this retrospective observational study, we analyzed data from chronic hepatitis C patients who were treated in six centers in the South of Brazil, located in five different cities of the State of Paraná: Curitiba, Londrina, Cascavel, Ponta Grossa, and Maringá. The study was carried out in accordance with the ethical principles of the Declaration of Helsinki and was approved by the local Ethics Committee on Human Research.
Data were collected from databases from each center, which contain all the individual patient records. The sample comprised adult patients (≥18 years) diagnosed with chronic HCV infection who concluded or discontinued any second generation DAA treatment before April 2017. Patients were included regardless of genotype, prior treatment, or liver fibrosis stage. The choice, administration, and management of each patient's treatment was the responsibility of the centers, according to the national guidelines.4
The primary effectiveness outcome was SVR 12 weeks or more after the end of treatment (SVR12), which is defined as undetectable HCV by polymerase chain reaction (PCR). Secondary effectiveness outcomes included rapid virological response (RVR; defined as undetectable HCV RNA after four weeks of therapy) and end of treatment response (EOTr; defined as undetectable HCV RNA at treatment completion), both measured using PCR tests. Patients that completed therapy but did not have any SVR results (i.e., missing data or lost to follow up) were excluded from the analysis.
Clinical information collected at baseline included age, sex, weight, presence/absence of cirrhosis, prior treatment information, HCV genotype, viral load, liver biopsy information, intended treatment, comorbidities (hepatitis B, HIV), other clinical information (prior transplantation or hepatocarcinoma), and treatment discontinuation due to adverse events (tolerability outcome).
Statistical analysisBaseline categorical variables are described as absolute and relative frequencies, while continuous variables are reported as medians and standard deviations. Analysis was carried out using the chi-square test and logistic regression to identify variables significantly associated with SVR (e.g., baseline parameters: treatment option, duration, sex, age, cirrhosis, prior treatment, and others). Bivariate logistic regression results are presented as odds ratios (OR) and their 95% confidence intervals (CI). A p-value less than 0.05 was considered statistically significant in all cases. Analysis was performed using SPSS Statistics version 24 (IBM SPSS, Chicago, IL, USA) and StatSoft Statistica version 10.
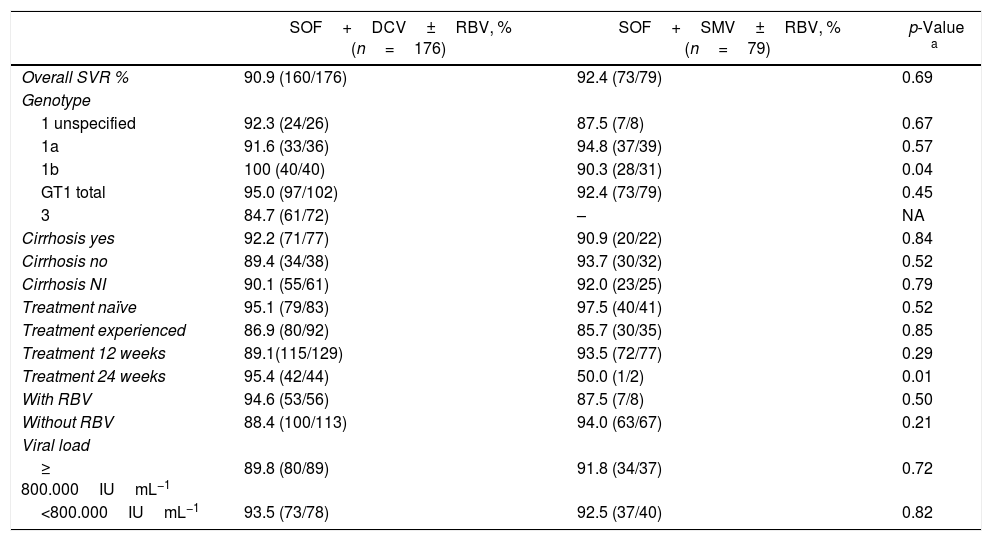
ResultsBaseline characteristicsIn the six centers, 363 patients were retrieved for this observational study, although 61 were excluded due to lost follow-up and another six due to missing data (e.g., no treatment information). Data from 296 patients were used for per protocol analysis. The mean age of the participants was 57.8±10.8 years and 56% of the sample were males. In terms of treatment, 59% of patients were treated with sofosbuvir plus daclatasvir, 26% received sofosbuvir plus simeprevir, and 15% received other DAA therapy options. In total, 34% of patients received ribavirin together with the DAA treatment. Genotypes 1 (GT1; 66%) and 3 (GT3; 27%) were the most frequent genotypes observed, and 48% of the patients were treatment experienced. Baseline characteristics of patients that received the two most prevalent treatments (n=255), sofosbuvir with daclatasvir, and sofosbuvir with simeprevir, are shown in Table 1.
Baseline demographic and clinical characteristics.
| Characteristic | SOF+DCV±RBV (n=176) | SOF+SMV±RBV (n=79) | Total (n=255) |
|---|---|---|---|
| Male, n (%) | 103 (58.5%) | 38 (48.1%)a | 141 (55.2%)a |
| Mean (SD) age, years | 58.1 (±10.3) | 57.1 (±12.4) | 57.8 (±10.8) |
| HCV genotype | |||
| 1 total | 102 (57.9%) | 79 (100.0%) | 198 (66.8%) |
| 1 unspecified | 26 (14.7%) | 8 (10.1%) | 34 (13.3%) |
| 1a | 36 (20.4%) | 39 (49.3%) | 75 (29.4%) |
| 1b | 40 (22.7%) | 31 (39.2%) | 71 (27.8%) |
| 3 | 72 (41.0%) | – | 72 (28.2%) |
| Missing data | 2 (1.1%) | 1 (1.2%) | 3 (1.1%) |
| Viral load≥800,000IUmL−1, n (%) | 89 (50.5%) | 37 (46.8%) | 126 (49.4%) |
| Missing data | 9 (5.1%) | 2 (2.5%) | 11 (4.3%) |
| Treatment experience, n (%) | 92 (52.2%) | 35 (44.3%) | 127 (49.8%) |
| Missing data | 1 (0.5%) | 3 (3.7%) | 4 (1.5%) |
| Fibrosis stage, n (%) | |||
| F0–F3 | 38 (21.5%) | 32 (40.5%) | 70 (27.4%)b |
| F4 (cirrhotic) | 77 (43.7%) | 22 (27.8%) | 99 (38.8%)b |
| Missing data | 61 (34.6%) | 25 (31.6%) | 86 (33.7%) |
| Liver transplantation | 5 (2.8%) | 3 (3.7%) | 8 (3.1%) |
| Treatment duration, n (%) | |||
| 12 weeks | 129 (73.0%)a | 77 (97.4%) | 206 (80.7%)a,b |
| 24 weeks | 44 (25.0%) | 2 (2.5%) | 46 (18.0%)b |
| Addition of RBV, n (%) | 56 (31.8%) | 8 (10.1%) | 64 (25.0%) |
| Co-infection | |||
| Hepatitis B virus (HBV) | 4 (2.2%) | 1 (1.2%) | 5 (1.9%) |
| Human immunodeficiency virus (HIV) | 10 (5.6%) | – | 10 (13.9%) |
p<0.05 (comparison between number of patients in each group that received sofosbuvir with daclatasvir versus sofosbuvir with simeprevir). Abbreviations: DAA, direct acting antivirals; DCV, daclatasvir; IFN, interferon; HCV, hepatitis C virus; LED, ledipasvir; RBV, ribavirin; SD, standard deviation; SMV, simeprevir; SOF, sofosbuvir.
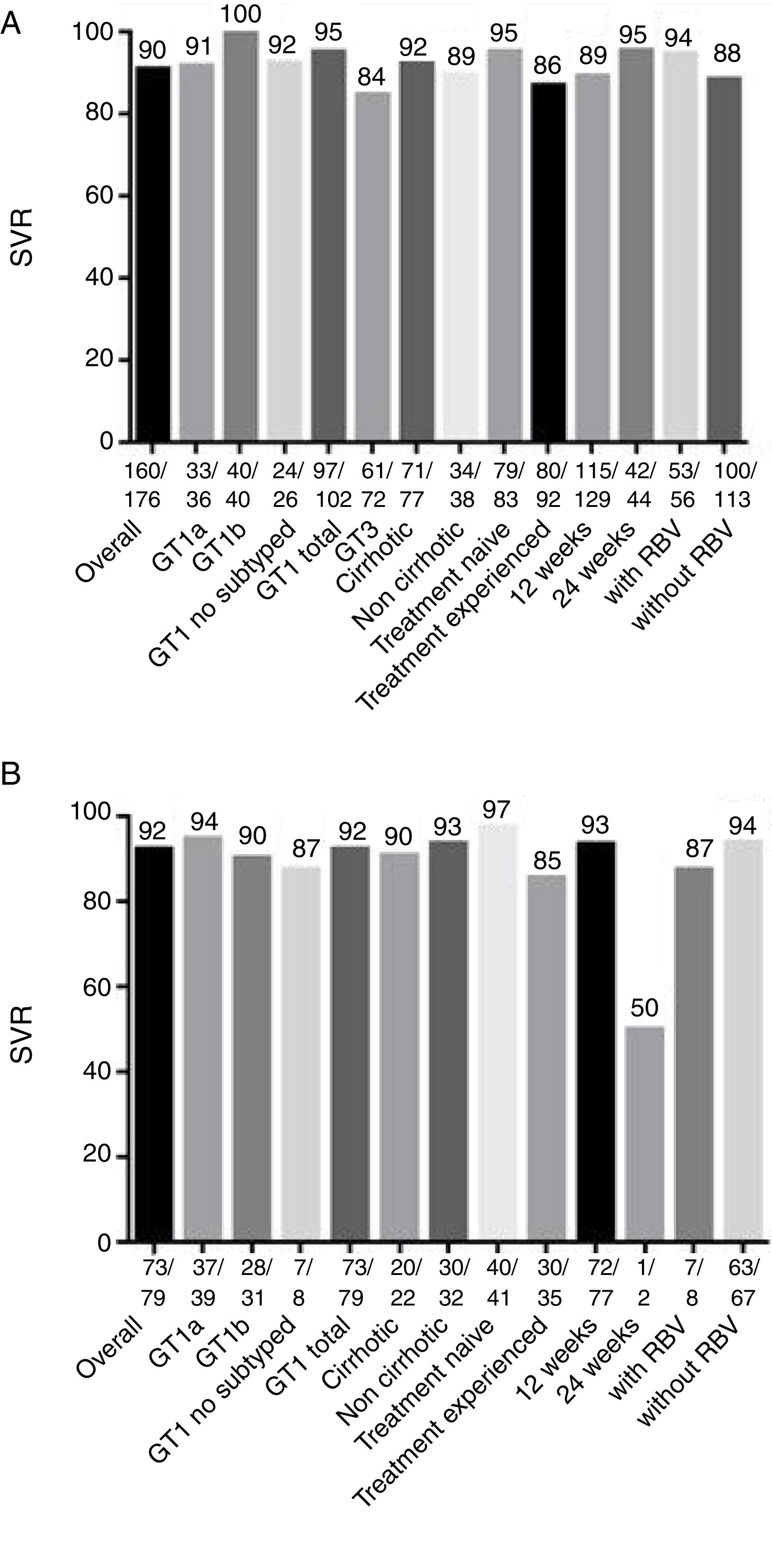
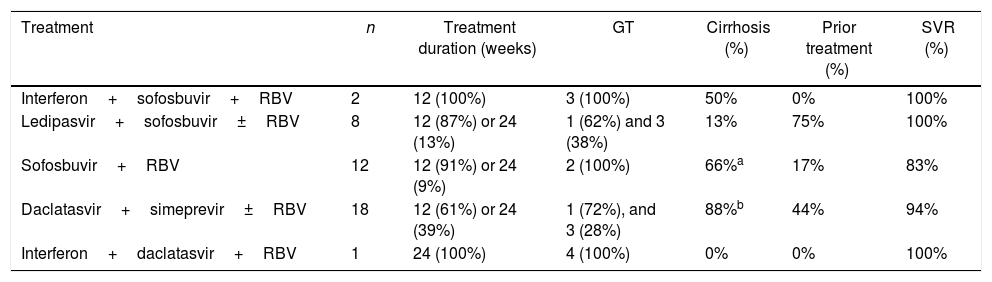
SVR was achieved by 160 patients (90%) of the 176 patients who were treated with sofosbuvir and daclatasvir (Fig. 1A and Table 2). This treatment was recommended for patients with GT 1 and 3; GT3 had lower SVR rates compared to patients infected with GT1 (95% versus 84%, p<0.05). All GT3 patients treated for 24 weeks achieved treatment response, compared to only 85% of GT3 patients treated for 12 weeks achieved SVR (although in this case p>0.05, more details in Supplementary Material 1). However, there was no statistically significant difference (p>0.05) in SVR rates in any of the other sub-analysis, including the use of sofosbuvir and daclatasvir with or without ribavirin (94% versus 88%), presence or absence of cirrhosis (92% versus 89%), prior treatment (95% versus 86%, respectively for treatment naïve and experienced patients), and treatment duration (95% versus 89%, respectively for 24 and 12 weeks of treatment).
Analysis of sustained virological response (SVR) and comparison of patients treated with sofosbuvir and daclatasvir versus sofosbuvir and simeprevir.
| SOF+DCV±RBV, % (n=176) | SOF+SMV±RBV, % (n=79) | p-Value a | |
|---|---|---|---|
| Overall SVR % | 90.9 (160/176) | 92.4 (73/79) | 0.69 |
| Genotype | |||
| 1 unspecified | 92.3 (24/26) | 87.5 (7/8) | 0.67 |
| 1a | 91.6 (33/36) | 94.8 (37/39) | 0.57 |
| 1b | 100 (40/40) | 90.3 (28/31) | 0.04 |
| GT1 total | 95.0 (97/102) | 92.4 (73/79) | 0.45 |
| 3 | 84.7 (61/72) | – | NA |
| Cirrhosis yes | 92.2 (71/77) | 90.9 (20/22) | 0.84 |
| Cirrhosis no | 89.4 (34/38) | 93.7 (30/32) | 0.52 |
| Cirrhosis NI | 90.1 (55/61) | 92.0 (23/25) | 0.79 |
| Treatment naïve | 95.1 (79/83) | 97.5 (40/41) | 0.52 |
| Treatment experienced | 86.9 (80/92) | 85.7 (30/35) | 0.85 |
| Treatment 12 weeks | 89.1(115/129) | 93.5 (72/77) | 0.29 |
| Treatment 24 weeks | 95.4 (42/44) | 50.0 (1/2) | 0.01 |
| With RBV | 94.6 (53/56) | 87.5 (7/8) | 0.50 |
| Without RBV | 88.4 (100/113) | 94.0 (63/67) | 0.21 |
| Viral load | |||
| ≥ 800.000IUmL−1 | 89.8 (80/89) | 91.8 (34/37) | 0.72 |
| <800.000IUmL−1 | 93.5 (73/78) | 92.5 (37/40) | 0.82 |
Note: Some groups have different number of patients compared to total number of patients as it occurred missing data in some baseline parameters presented in Table 1.
RVR was obtained by 74 of 78 patients (94%), while EOTr was achieved by 90% (64 of 71). More detail regarding secondary outcomes is available in Supplementary Material 3.
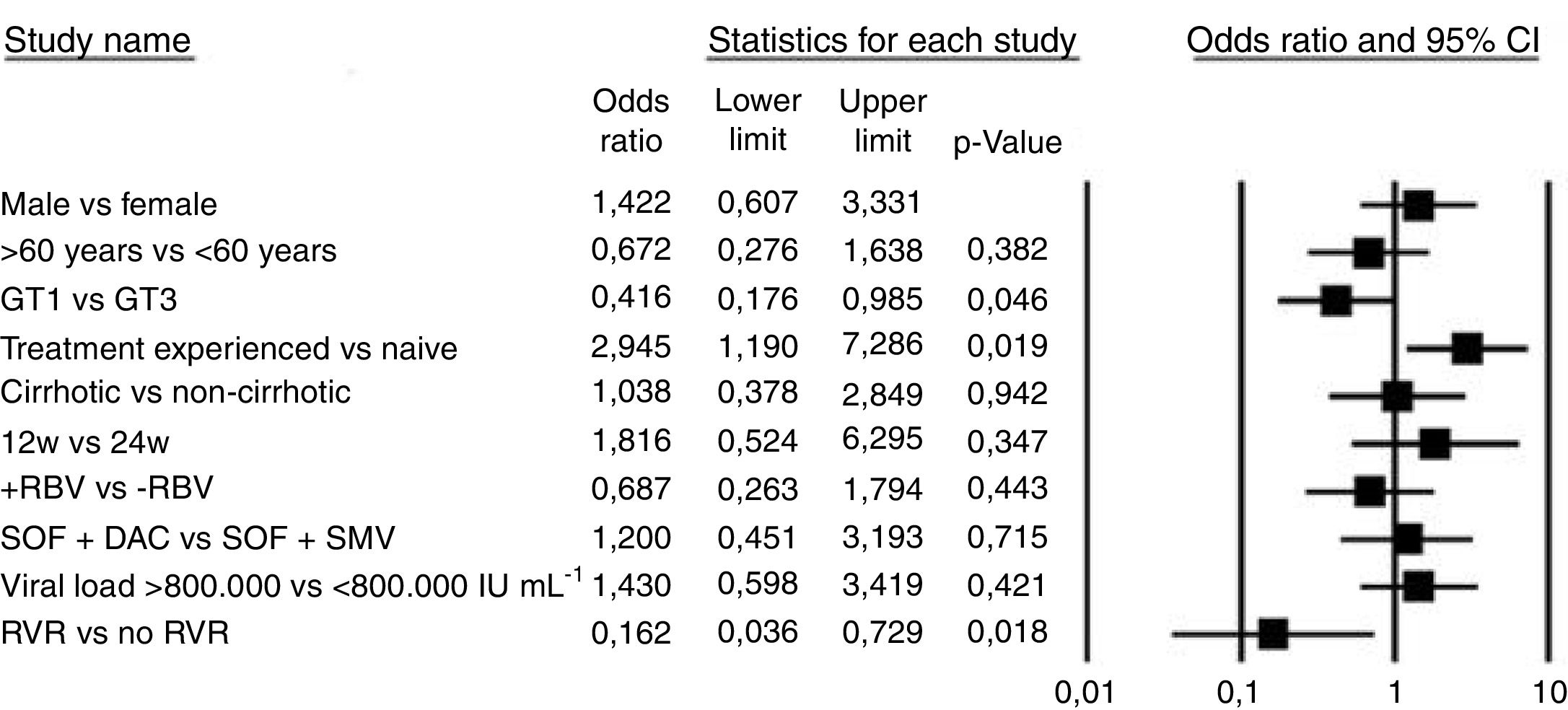
Sub-cohort analysis of patients receiving sofosbuvir with simeprevirOf the 79 patients that received sofosbuvir with simeprevir, 73 (92%) achieved SVR (Fig. 1B and Table 2). This DAA combination was only administered to HCV GT 1 patients; SVR rate was 94% for subtype 1a, 90% for subtype 1b, and 87% for unspecified GT 1 subtype. Other sub-analysis included prior treatment (97% versus 85% for treatment naïve and experienced patients). Use or non-use of ribavirin (87% versus 94%), and presence or absence of cirrhosis (90% versus 93%). There were no statistically significant differences (p>0.05), except for treatment duration (93% versus 50%, respectively for 12 and 24 weeks of treatment); however, this finding should be interpreted with caution as only two patients received treatment for 24 weeks.
RVR was achieved by 42 of 47 patients (89%), while EOTr was obtained by 100% of the patients where data was available (19 of 19). More detail regarding secondary outcomes is available in Supplementary Material 3.
Comparative effectiveness of sofosbuvir with daclatasvir versus sofosbuvir with simeprevirOverall SVR rates were similar for both treatments (Table 2). For GT 1, sofosbuvir with daclatasvir had an SVR rate of 95%, compared to 92% for sofosbuvir with simeprevir (p>0.05), however, for GT 1b, sofosbuvir with daclatasvir was more effective (p<0.05). No statistically significant differences between therapies were observed in terms of prior treatment, cirrhosis (Supplementary Material 2 presents more details about SVR regarding genotype and presence/absence of cirrhosis), or additional use of ribavirin. Further, the sub-analyses did not produce statistically significant results (p>0.05 in all analysis). Treatment in patients receiving therapy for 24 weeks was more effective in those who received sofosbuvir with daclatasvir; however, as only two patients received sofosbuvir with simeprevir for 24 weeks, this result should again be interpreted with caution.
Patients treated with other DAA optionsIn total, 41 patients were treated with different DAAs from those previously described; 18 received daclatasvir with simeprevir, 12 received sofosbuvir with ribavirin, two were treated with pegylated interferon with sofosbuvir and ribavirin, eight were treated with ledipasvir with sofosbuvir, and one received pegylated interferon with daclatasvir and ribavirin. Three of these patients did not achieve SVR (one treated with daclatasvir and simeprevir, and two with sofosbuvir with ribavirin). Baseline data and results for these patients are detailed in Table 3.
Response rates for patients treated with other DAA options.
| Treatment | n | Treatment duration (weeks) | GT | Cirrhosis (%) | Prior treatment (%) | SVR (%) |
|---|---|---|---|---|---|---|
| Interferon+sofosbuvir+RBV | 2 | 12 (100%) | 3 (100%) | 50% | 0% | 100% |
| Ledipasvir+sofosbuvir±RBV | 8 | 12 (87%) or 24 (13%) | 1 (62%) and 3 (38%) | 13% | 75% | 100% |
| Sofosbuvir+RBV | 12 | 12 (91%) or 24 (9%) | 2 (100%) | 66%a | 17% | 83% |
| Daclatasvir+simeprevir±RBV | 18 | 12 (61%) or 24 (39%) | 1 (72%), and 3 (28%) | 88%b | 44% | 94% |
| Interferon+daclatasvir+RBV | 1 | 24 (100%) | 4 (100%) | 0% | 0% | 100% |
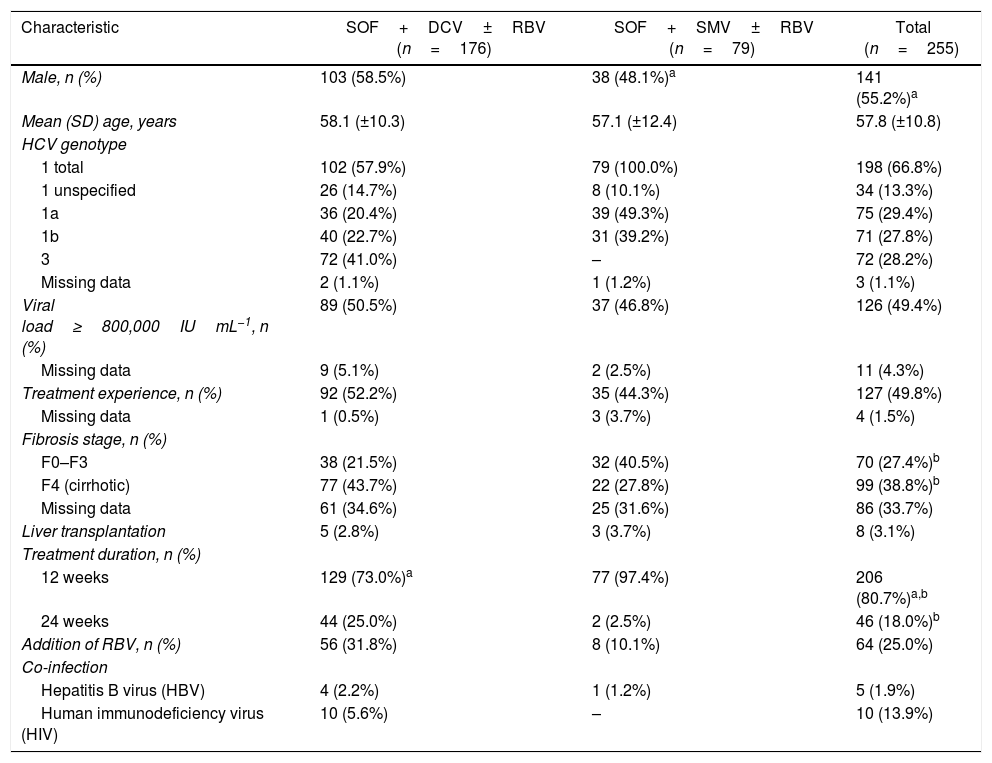
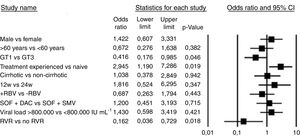
Bivariate logistic regression was used to assess the influence of several baseline parameters on treatment response (Fig. 2). Sex, age, cirrhosis, prior treatment, treatment duration, use of ribavirin, baseline viral load, and therapy choice were not associated with treatment success (p>0.05). The only two statistically significant predictors of treatment response (both p<0.05) were genotype 1 compared with genotype 3 (OR 0.41 [CI 0.17–0.98]) and RVR response (OR 0.16 [CI 0.03–0.72]).
Regarding tolerability, only three patients (1%) discontinued treatment due to adverse events, but all achieved SVR. The first was treated with pegylated interferon with sofosbuvir and ribavirin, and discontinued treatment due to epigastralgia. The second received sofosbuvir with daclatasvir, and suspended treatment due to gastric hemorrhage. The third also received sofosbuvir with daclatasvir but the reason for treatment discontinuation was not described.
DiscussionThe second generation DAAs evaluated in this observational study produced significant SVR rates. The results are in agreement with previous clinical trials and systematic reviews.12–14 As such, it appears that the findings obtained in controlled studies are being experienced in clinical practice in Brazil. Previous observational studies17–19 conducted in other Brazilian states have shown overall SVR rates of 93–100%, which is not very different from the findings of the present study (91.6%).
Besides the high response rates, some points must be noted. GT 3 appears to be the most difficult to treat and is the second most common genotype in Brazil (after genotype 1). However, only two treatment options are available in the country to date: sofosbuvir with daclatasvir and sofosbuvir with peginterferon alfa plus ribavirin. Previous observational studies have also shown a lower SVR rate for GT 3 patients.17,20–22 Other international guidelines, such as those of the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) recommend other treatment options for GT 3,7,8 such as sofosbuvir with velpatasvir, or elbasvir with grazoprevir. It is also important to mention that many of these patients received treatment of sofosbuvir with daclatasvir for a short duration (12 weeks), which may have influenced the SVR rate in these cases. Recently, the Brazilian government has approved the extension of the sofosbuvir with daclatasvir treatment to 24 weeks for cirrhotic patients.4 This, together with the addition of other drugs could increase the likelihood of cure for patients with GT 3.
GT 1, which is the most prevalent in Brazil, used to be the most difficult to treat genotype for many years.23,24 Second generation DAAs is effective for treating GT 1 infection, with SVR rates around 90%. In this study, patients with GT 1 presented higher SVR rates than those with GT 3 when treated with sofosbuvir and daclatasvir.
Few statistically significant differences were found between the factors predicting SVR. However, patients who achieved RVR had a greater likelihood of also achieving SVR. Other studies that performed similar analysis found that liver function and platelet count were factors associated with SVR.25–27
The stage of disease, i.e., fibrosis stage and presence/absence of cirrhosis, is recognized as an influence on SVR. Our analysis did not show this correlation, probably due to a considerable amount of missing data for this variable, and the difference in the number of patients in each treatment group. Nevertheless, patients with severe fibrosis should always be carefully monitored. A previous study has also suggested that liver fibrosis and HIV coinfection influences treatment outcome.28
Patients with GT 1 treated with sofosbuvir and daclatasvir had similar SVR rates than those treated with sofosbuvir and simeprevir. A pharmacoeconomic study analyzing these two treatment options, as well as other treatment options, could help health professionals to make clinical decisions regarding therapeutic choice.
Some limitations of our study should be noted. Caution should be taken when generalizing the results to other Brazilian regions and other countries, due to regional variations. Further, the absence of some information, especially fibrosis stage, may have compromised some of the analysis.
ConclusionsThe results of this observational study confirm that second generation DAAs are effective for the treatment of chronic hepatitis C patients in Southern Brazil, particularly for genotype 1. Genotype 3 appears to be the most difficult to treat, but even so, current SVR rates with second generation DAAs were higher than with previous therapies.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to express our thanks to the Brazilian Hepatology Society (Sociedade Brasileira de Hepatologia; SBH) for sharing the data collection table and for all research support.