To evaluate the cumulative incidence of dyslipidemia and fasting glucose impairment three years after initiating the first antiretroviral (ART) regimen and the association with the type of ART regimen in an AIDS outpatient clinic in Brazil.
MethodsRetrospective cohort of HIV-1 infected patients attending an outpatient HIV clinic in Vitoria, Brazil, between January/2010 and May/2011. Data, including blood pressure, dyslipidemia (high total cholesterol and low HDL-C), fasting glucose, and cardiovascular risk by Framingham Risk Score were abstracted from medical records from clinic visits six months prior and three years after starting ART. We assessed independent associated factors for dyslipidemia using multiple logistic regression.
ResultsFour hundred and ninety-eight patients on ART were studied. Median age was 45 years (interquartile range (IQR): 37–52), and median time since HIV diagnosis was 7.7 years (IQR: 3.8–10.0). The proportion of patients with dyslipidemia was 22.3% (95% CI: 18.6–25.9%) 36 months after ART initiation. Triglycerides levels >150mg/dL (55.2% vs. 25.4%, p=0.021) and high fasting glucose (5.8% vs. 2.3%, p=0.034) were diagnosed more frequently after ART use when compared to baseline values. Multiple logistic regression analysis has shown dyslipidemia to be associated with lopinavir/r use [OR=1.74 (95% CI: 1.12–2.86)].
ConclusionThese data show high chance of dyslipidemia after initiation of ART. Long-term follow-up will help identify the impact of ART on cardiovascular risk.
AIDS associated morbidity and mortality has substantially declined since the widespread use of highly active antiretroviral therapy (ART). Data from Brazil were among the first to show this finding.1 Survival time has increased significantly among adult Brazilian AIDS patients, which demonstrates the benefits of universal access to antiretroviral treatment in a developing country. On the other hand, non-AIDS conditions are emerging as significant causes of morbidity and mortality in the HIV-infected population. Increased rates of dyslipidemia and diabetes have been well documented,2,3 with multiple factors accounting for these findings, especially the use of antiretroviral drugs. HIV infection itself has been associated with decreases in low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, and progressive appearance of hypertriglyceridemia, likely related to Interferon alpha (IFNα) circulating levels in later stage disease.4 A significant association between decreasing CD4+ cell count and decreasing levels of HDL has been described, as well as an association between a history of AIDS-defining events and higher total cholesterol and triglyceride concentrations.2
The incidence of diabetes mellitus in HIV-infected men with ART exposure has been shown to be over four times than that seen in HIV-negative men in the Multicenter AIDS Cohort Study,5 after adjusting for body mass index and age. Insulin resistance has been reported in 13% of HIV-infected patients after a year of ART in a prospective observational cohort study.6 In HIV-infected women, diabetes mellitus incidence has been shown to be associated with and longer cumulative exposure to nucleoside reverse transcriptase inhibitors (NRTI).7
As non-AIDS morbidity has became more significant in recent years, there has been speculation whether HIV infection may be a marker for higher cardiovascular risk, or exposures unrelated to HIV or ART.8 HIV-infected patients, for example, usually have higher smoking rates, and usually need assistance to quit.8–10
Brazilian national guidelines for ART therapy in adults11 recommend routinely estimation of cardiovascular risk by use of Framingham Risk Score (FRS). Estimation of the risk for a cardiovascular event in ten years by the FRS is based on age, total cholesterol, HDL-cholesterol, systolic blood pressure and smoking status.12 It is designed for patients aged 20 years and older who do not have heart disease or diabetes. As few studies have reported the prevalence of these metabolic complications in our country,13–15 the aim of our study was to evaluate the cumulative incidence of dyslipidemia and fasting glucose impairment three years after starting ART and to investigate the association between ART and dyslipidemia in an AIDS outpatient clinic in Brazil.
MethodsThis was a retrospective cohort study of HIV-1 infected adult patients (18 years and older) attending an AIDS outpatient clinic at a University Hospital in Vitória, Brazil, between January 2008 and May 2011. This clinic is the second largest Specialized Assistance Service (SAS) in the city of Vitoria, working since the 1980s and is part of the national public network providing care for HIV-infected patients in Brazil.
Six hundred and seventy-nine patients were followed in this outpatient clinic during the study period. A total of 164 were excluded due to unavailability of lipid, glucose or FRS results before treatment, diagnosis of dyslipidemia before starting ART, or lost to follow-up. The majority of the excluded patients were aged less than 30 years and thus no lipid profile had been requested. The remaining 515 patients were included.
Demographic, behavioral and clinical data including blood pressure measurements, total and HDL cholesterol, fasting glucose, and FRS were abstracted from medical records and interviews with all patients, from the last visit (3–6 months) prior to and three years following initiation of ART. Information on time on ART, type of ART, smoking status, and lipid lowering therapy was also collected. LDL-cholesterol was calculated using Friedwald's formula in individuals with triglycerides ≤400mg/dL. When TGL >400, LDL cholesterol was not available.
Blood samples were collected after 12h fasting, according to the usual laboratory protocol. All patients included in the analyses were on antiretroviral therapy according to Brazilian Consensus of Antiretroviral Therapy for Adults guideline.11 Only patients on their first ARV regimen were studied. In case of ARV regimen change the regimen used for at least 70% of the study period was considered.
Results of HDL-cholesterol were stratified as normal or low (<40mg/dL in men and <50mg/dL in women); total cholesterol was deemed normal or high (>200mg/dL), LDL cholesterol as normal or high (>160mg/dL), and triglycerides as normal or high (>150mg/dL), according to the Brazilian Cardiology Society guidelines.16 Impaired fasting glucose and diabetes were defined if measurements of fasting glucose levels were >99mg/dL and >126mg/dL, respectively. Patients with low HDL cholesterol and high total cholesterol levels were grouped as having “dyslipidemia”.
Patient data were entered into a database and analyzed using SPSS, version 17 for Windows (Chicago, IL, USA). Standard descriptive analyses were performed including frequency distributions for categorical data and calculation of medians and interquartile ranges for continuous variables. Univariate analyses to assess factors associated with dyslipidemia were tested using Chi-square and Fisher's Exact Test, and t-test and analysis of variance were used for testing differences between means. Odds ratio (OR) was used as a measure of association, estimated with a 95% CI. Independent risk factors for “dyslipidemia” were assessed through multiple logistic regression with 15% as the critical p-value for variable entry and 10% as the criterion for variable elimination.
The study protocol was submitted and approved by the Escola de Ciências da Saúde da Santa Casa de Misericordia institutional review board (# 048/2007).
ResultsFive hundred and fifteen (72.1%) patients on ART had lipid and glucose data available before and after ARV treatment and were included in the study. Among them 17 (3.3%) had been diagnosed with dyslipidemia before ART and were not included. Of the remaining 498 patients, 309 (58.9%) were male and 301 (60.4%) Caucasian. Median age was 45 years at 2010/2011 visit (interquartile range [IQR]: 37–52), and median time since HIV diagnosis was 7.7 years (IQR: 3.8–10.0). The cohort's median age is few years older than the usual age in our region as many young patients had no lipid profile measured before ART. The cumulative incidence of dyslipidemia was 22.3% (95% CI: 18.6–25.9%) after three years of ART. There was no difference between genders.
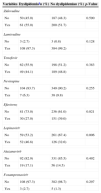
Table 1 shows demographic, behavioral and clinical characteristics. There were no baseline differences between patients who developed and who did not develop dyslipidemia on ART. The most frequently prescribed nucleoside reverse transcriptase inhibitors (NRTI) backbone was lamivudine–zidovudine, used by 53.1% of patients. Stavudine was no longer used, and tenofovir–lamivudine, abacavir–lamivudine and didanosine–lamivudine were used by the remaining patients. Lopinavir/r (46.8% vs. 32.6%, p=0.006) was associated with increased odds of dyslipidemia while efavirenz (27.0% vs. 39.0%, p=0.021) was associated with decreased odds (Table 2).
Incident dyslipidemia by baseline demographic, behavioral and clinical characteristics, HIV patients on antiretroviral therapy, Vitoria, Brazil (n=498).
| Variables | Dyslipidemiaan (%) | No dyslipidemian (%) | p-Value |
|---|---|---|---|
| Age (years) | |||
| 18–29 | 4 (3.6) | 24 (6.2) | 0.559 |
| 30–49 | 72 (64.9) | 239 (62.1) | |
| 50 or more | 35 (31.5) | 122 (31.7) | |
| Gender | |||
| Male | 62 (55.9) | 247 (63.8) | 0.149 |
| Female | 49 (44.1) | 140 (36.2) | |
| Education (years) | |||
| Up to 4 | 17 (15.3) | 39 (10.2) | 0.172 |
| More than 4 | 94 (84.7) | 344 (89.8) | |
| Tobacco use | |||
| Yes | 34 (31.5) | 139 (37.5) | 0.306 |
| No | 74 (68.5) | 232 (62.5) | |
| Nadir CD4+ (cells/mL) | |||
| Up to 200 | 68 (61.3) | 224 (59.1) | 0.742 |
| More than 200 | 43 (38.7) | 155 (40.9) | |
| Hypertension | |||
| Yes | 23 (20.7) | 71 (18.5) | 0.586 |
| No | 88 (79.3) | 312 (81.5) | |
| Previous diabetes | |||
| Yes | 3 (2.7) | 9 (2.3) | 0.945 |
| No | 108 (97.3) | 375 (97.7) | |
| HCV infection | |||
| Yes | 5 (4.5) | 24 (6.3) | 0.648 |
| No | 106 (95.5) | 357 (93.7) | |
Incident dyslipidemia by antiretroviral therapy use, HIV patients, Vitoria, Brazil (n=498).
| Variables | Dyslipidemiaan (%) | No dyslipidemian (%) | p-Value |
|---|---|---|---|
| Zidovudine | |||
| No | 50 (45.0) | 187 (48.3) | 0.590 |
| Yes | 61 (55.0) | 200 (51.7) | |
| Lamivudine | |||
| No | 3 (2.7) | 3 (0.8) | 0.128 |
| Yes | 108 (97.3) | 384 (99.2) | |
| Tenofovir | |||
| No | 62 (55.9) | 198 (51.2) | 0.383 |
| Yes | 49 (44.1) | 189 (48.8) | |
| Nevirapine | |||
| No | 104 (93.7) | 349 (90.2) | 0.255 |
| Yes | 7 (6.3) | 38 (9.8) | |
| Efavirenz | |||
| No | 81 (73.0) | 236 (61.0) | 0.021 |
| Yes | 30 (27.0) | 151 (39.0) | |
| Lopinavir/r | |||
| No | 59 (53.2) | 261 (67.4) | 0.006 |
| Yes | 52 (46.8) | 126 (32.6) | |
| Atazanavir/r | |||
| No | 92 (82.9) | 331 (85.5) | 0.492 |
| Yes | 19 (17.1) | 56 (14.5) | |
| Fosamprenavir/r | |||
| No | 108 (97.3) | 382 (98.7) | 0.297 |
| Yes | 3 (2.7) | 5 (1.3) | |
Median FRS changed from 1 (IQR: 1–2) before ART to 3 (IQR: 1–9.5) after therapy (p<0.001). Table 3 shows the distribution of lipids, glucose and FRS before and after ART. All means were significantly higher in the second measurement.
Serum lipid and fasting glucose levels before and after ART initiation, HIV patients Vitoria, Brazil.
| Before ART mean (SD) | After ART mean (SD) | p-Value | |
|---|---|---|---|
| Cholesterol (mg/dL) | 163.0 (36.3) | 194.9 (41.3) | 0.001 |
| HDL-C (mg/dL) | 41.8 (12.9) | 44.5 (13.1) | 0.001 |
| LDL-C (mg/dL) | 93.2 (29.7) | 113.8 (35.4) | 0.001 |
| Triglycerides (mg/dL) | 138.1 (81.3) | 191.8 (76.0) | 0.001 |
| Fasting glucose (mg/dL) | 85.2 (16.7) | 98.4 (21.1) | 0.001 |
| FRS median (IQR) | 1 (1–2) | 3 (1–9.5) | 0.001 |
Triglyceride levels were high in 25.4% of patients before treatment increasing to 55.2% after treatment (p=0.021). High triglyceride levels were associated with age higher than 50 years (36.5% vs. 27.9%, p=0.039) and with lopinavir/r use (41.3% vs. 30.2%, p=0.009). Impaired fasting glucose was observed in 42 patients (8.4%) before ART and in 183 patients (36.7%) after ART. Diabetes was observed in 2.4% (95% CI: 1.0–3.6) patients before ART and in 5.8% (95% CI: 3.2–7.0) after three years of ART use (p=0.034). Impaired fasting glucose was also more frequent in patients aged higher than 50 years (53.3% vs. 30.8%, p=0.010), and in patients co-infected with hepatitis C virus (16.7% vs. 5.6%, p=0.032). No association was found with CD4 nadir.
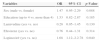
In the final regression model, lopinavir/r use [OR=1.74 (95% CI: 1.12–2.86)] remained significantly and independently associated with dyslipidemia (high cholesterol and low HDL-C) after ART (Table 4).
Variables independently associated with dyslipidemia, HIV patients on antiretroviral therapy, Vitoria, Brazil (n=494).
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| Sex (male vs. female) | 1.47 | 0.95–2.29 | 0.088 |
| Education (up to 4 vs. more than 4) | 1.53 | 0.82–2.87 | 0.185 |
| Lamivudine (yes vs. no) | 0.28 | 0.05–1.45 | 0.130 |
| Efavirenz (yes vs. no) | 0.76 | 0.44–1.31 | 0.314 |
| Lopinavir/r (yes vs. no) | 1.68 | 1.12–2.2.78 | 0.040 |
Low HDL-cholesterol, defined as HDL <40mg/dL for men and <50mg/dL for women was found in 58.1% of HIV-infected patients prior to initiating ART. Although a modest but significant increase on HDL cholesterol mean was observed after ART, the proportion of patients with low HDL remains almost the same as it was before ART. These results are consistent with advanced HIV disease.17 Rose et al. suggested that HIV infection is associated with modified HDL metabolism re-directing cholesterol to the apo B-containing lipoproteins and likely reducing the functionality of reverse cholesterol transport.18 Higher activity of cholesterol ester transfers protein (CETP) correlated negatively with levels of HDL-C and is probably associated with higher risks of coronary artery disease especially in patients with hypertriglyceridemia.19
We also observed high triglyceride levels in 55.2% of our patients after treatment initiation, a twofold higher proportion than that observed prior to ART (25.4%). Ceccato et al. also reported a high prevalence of hypertriglyceridemia and low HDL (32.4%) among HIV patients after ART in a reference center in Brazil.20 High cholesterol levels were observed among 45.8% of treated patients, threefold higher than what was observed before treatment and it was associated with lopinavir/r use. A meta-analysis of 15 clinical trials enrolling a total of 6368 patients, designed to evaluate effects of first line antiretroviral therapy on lipids levels, showed that lopinavir/r and fosamprenavir/r were associated with greater elevations in total cholesterol and triglycerides.21 Our findings are consistent with a small effect of ART on absolute changes in HDL levels from baseline to three years of follow up.
Hypertriglyceridemia in untreated HIV-infected patients may be a consequence of a systemic inflammatory response against persistent viral infection and it correlates with serum interferon-alpha levels.22 In Brazil, lopinavir/r is the first choice protease inhibitor, thus highly used by our patients. Others authors have shown that four weeks of lopinavir/r use are enough to significantly raise lipid levels.23 Brazilian treatment guidelines are currently being revised, so other protease inhibitors may be added as first choice options, especially given knowledge of associated elevations in lipids.
Impaired fasting glucose was present in a significantly higher proportion of patients after ART initiation. A study has shown a modest association between untreated HIV and insulin resistance, mediated by inflammatory cytokines.24 However, Blanco et al. have reported that impaired glucose tolerance in treatment-experienced patients is higher (10–25%) than among treatment-naïve patients (5–10%), especially in those receiving protease inhibitors.25 Some evidence links protease inhibitors to the pathogenesis of insulin resistance, with acute inhibition of GLUT4-mediated glucose transport, and defective insulin signaling.26 We did not find an association of impaired glucose tolerance or diabetes to any specific ART type, possibly due to the modest sample size.
Our results underscore the association between impaired fasting glucose and elevated triglycerides with age higher than 50 years old. Median age of our patients was only 45 years and these metabolic disturbances shall get worse as this population age. Projections of global mortality from the World Health Organization predict that ischemic heart disease will be among the first three causes of death in the HIV population in low income countries.27 Active prevention, immediate diagnosis and management of cardiovascular risk factors, like the metabolic disturbances here described, must be standard of care in outpatient clinics.
We have not found discrepant variations in median FRS before and after treatment. Although the FRS has not been specifically validated in HIV-positive patients, it has been applied since no other option is already available. Nevertheless, some speculate that it may underestimate the cardiovascular risk, especially in smokers.28 All but two of our patients on statin therapy were using pravastatin 40mg/day. Pravastatin is a weak statin, and most of the time it was prescribed it was not available for the patients in need. Rosuvastatin has been proven to be more effective in HIV patients29 and may further improve lipid profiles in our patients, when available. Seventy-two patients were prescribed statins, but with so irregular use, that the impact on lipids could not be evaluated.
We observed a high prevalence of tobacco use among our patients (36%) that varies little (and this value does not change significantly) between the two Framingham time points (before and after ART). Tobacco use in our patient population was more than one and half times higher than the observed use among the adult population in Vitoria30 and even higher than the observed smoking habit among HIV-infected patients in Sao Paulo.31 We need to enhance our efforts to assist HIV-infected patients to quit smoking.
We acknowledge other limitations of the study: retrospective cohort study has limitations to analyze the effects of therapy in HIV infected patients. The issue of whether ART affects dyslipidemia is best addressed by prospective studies with HIV-infected patients either receiving or initiating therapy. Also, we did not have access to data regarding excluded patients and it might have caused bias because we cannot say if these patients are different or how different they are from the included patients. Another limitation is that given the low prevalence of some risk factors in this sample, it is possible that the number of patients studied was not sufficient to find statistical association between some independent variables and dyslipidemia.
In conclusion, we found frequent lipid and glucose perturbations in HIV-infected patients on ART, which may worsen over time. Infectious disease doctors, despite still looking at HIV viral load need to act as primary care physicians strongly encouraging their patients to quit smoking, pursuing the best goal on blood pressure control and taking a closer look on lipids and glucose levels. This must be a priority in the care of those patients especially as this population ages. Brazilian outpatient clinics need to focus more on those controls although increasing prevalence of cardiovascular risk factors will overshadow the amazing benefits of antiretroviral therapy.
Financial supportThis research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Conflict of interestWe declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
We wish to thank Dr. George Rutherford (UCSF) for reviewing the paper and his helpful editorial comments.