Several studies have demonstrated that piperacillin/tazobactam produces a false-positive result for the galactomannan antigen test. However, the most recent literature has demonstrated that this interaction is no longer a concern. There is little information regarding the drug–laboratory interaction with the generics of piperacillin/tazobactam or other broad-spectrum beta-lactams, such as ceftaroline, doripenem, imipenem/cilastatin, and meropenem. The purpose of this study was to determine if a drug–laboratory interaction exists with these antibiotics. Tests showed that one lot of imipenem/cilastatin by Hospira Healthcare India Private Limited produced a false-positive result for the galactomannan antigen test. All other medications tested, including piperacillin/tazobactam from seven manufacturers and imipenem/cilastatin by Hospira Inc., did not produce positive results. Since the reason for this drug–laboratory interaction with imipenem/cilastatin is unknown, more studies are needed to further investigate this interaction. Providers also should be educated of these findings: no drug–laboratory interaction with piperacillin/tazobactam and a possible drug–laboratory interaction with imipenem/cilastatin (Hospira Healthcare India Private Limited).
Patients with invasive Aspergillus infections have a high mortality rate; therefore, early diagnosis is important. The galactomannan antigen test, marketed as the Platelia™ Aspergillus Ag (Bio-Rad), is a noninvasive tool used with other diagnostic tests for the early identification of invasive Aspergillus infections. This antigen test works through detection of galactomannan, an antigen found in the cell wall of Aspergillus species.1 Limitations of this test include low sensitivity (50–80%) and cross-reaction with other fungi (e.g., Penicillium species, Histoplasma species, and Blastomyces species).1,2
Certain penicillin antibiotics have been reported to produce a false-positive galactomannan test, likely because they are derived from Penicillium species. Although a few reports have shown that the intravenous formulations of amoxicillin and amoxicillin/clavulanate (not available in the United States) produce a false-positive result for the galactomannan antigen test,1,3,4 most of the data demonstrating this drug–laboratory interaction is with brand name piperacillin/tazobactam (Zosyn®, Wyeth).1,3,5–12 In addition, researchers have found that the degree of positivity varies among batches of piperacillin/tazobactam and is dependent upon the time of the phlebotomy.5,7–9,11–13 Most of these in vitro and in vivo studies were conducted prior to September 2009, when generic piperacillin/tazobactam became available.14 As a result, little information is available regarding the drug–laboratory interaction with the generic versions of piperacillin/tazobactam and other broad-spectrum antibiotics. These broad-spectrum antibiotics are often used to empirically treat immunocompromised patients, the population in which this galactomannan antigen test is most commonly performed. Because positive galactomannan antigen tests may result in the overtreatment of these patients, broad-spectrum antibiotics should be tested for this interaction.
The aim of this study was to screen whether the six generic brands of piperacillin/tazobactam that are available in the United States, as well as other broad-spectrum beta-lactam antibiotics, produce a false-positive result for the galactomannan antigen test.
A vial of each antibiotic of interest available in the United States was obtained from the manufacturers, and pure chemical powders were ordered for comparison. Pure piperacillin and tazobactam powders were included in this study to investigate whether this interaction is due to the chemical itself or related to manufacturing processes. Antibiotics tested included piperacillin [pure chemical, Tokyo Chemical Industry (Lot IK2QM-BM, Exp NA)], tazobactam [pure chemical, Chem-Impex Int’l (Lot TB00N110629, Exp 6/28/2014)], piperacillin/tazobactam [Zosyn®, Wyeth (Lot AH1C/11, Exp 4/2015); six generics: Apotex (Lot 500C003, Exp 1/2014), APP (Lot 2C08TN, Exp 2/2015), Auromedics (Lot PT0212001-A, Exp 2/2014), Hospira (Lot 110278M, Exp 11/1/2013), Sagent (Lot PT0211008-A, Exp 8/2013), and Sandoz (Lot BY0110, Exp 9/2013)], imipenem/cilastatin [Primaxin®, Merck (Lot H007317, Exp 3/2014); two generics, APP (Lot 0013D21, Exp 4/2014) and Hospira (Batch 1, Lot 613C015, Exp 12/2013; Batch 2, Lot 613C025, Exp 8/2013)], meropenem [Merrem®, AstraZeneca (Lot JX109, Exp 2/2015)], doripenem [Doribax®, Ortho-McNeil (Lot ALZT600, Exp 11/2013)], and ceftaroline [Teflaro™, Forest (Lot 0012D16, Exp 11/2013)]. Antibiotic powders were measured and divided into aliquots representing the reconstituted concentration per the manufacturers’ package inserts when 1mL of diluent was added (i.e., 225mg/mL of piperacillin/tazobactam, 50mg/mL of imipenem/cilastatin, 50mg/mL of meropenem, 50mg/mL of doripenem, and 30mg/mL of ceftaroline).
Presence of the interaction was tested utilizing the Platelia™ Aspergillus Ag (Bio-Rad) kit (Kit 1, Prod# 62793, Lot 9J0030, Exp 11/30/2010; Kit 2, Prod# 62794, Lot 2F0017, Exp 6/5/2013) according to standard methods. Two kits were utilized for this study; one expired kit was used for a practice run while the results of this study were produced and reported using the in-date kit. All tests were run in quadruplicate between December 2012 and April 2013. For any medication with a positive initial result, four dilutions were subsequently tested to mimic drug concentrations in the body. Dilutions included the average peak serum concentration in the body according to the manufacturers’ package inserts plus one dilution above and two dilutions below that concentration. Dilutions were first tested in normal saline and then in human serum (drawn from the principle investigator). Antibiotics were diluted in serum to evaluate the drug–laboratory interaction in a clinical sample.
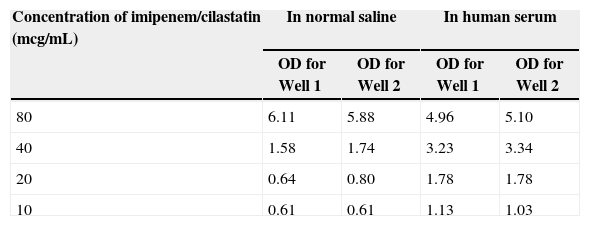
Imipenem/cilastatin by Hospira was the only medication that produced a positive galactomannan antigen test. All other reconstituted medications tested (including imipenem/cilastatin from two other manufacturers) did not produce a positive result. For imipenem/cilastatin by Hospira, the reconstituted medication (i.e., 50mg/mL), four dilutions in normal saline (i.e., 10, 20, 40, and 80mcg/mL), and four dilutions in human serum (i.e., 10, 20, 40, and 80mcg/mL) produced positive antigen tests. The optical density (OD) indices of the reconstituted medication were 2.96 and 2.97. Similar results were found with the four dilutions in both normal saline and human serum (Table 1), and when utilizing both galactomannan antigen kits. When testing a second batch of imipenem/cilastatin by Hospira (i.e., the same medication with a different lot number), the reconstituted medication and four dilutions in normal saline tested negative (i.e., OD indices<0.5). The four dilutions of the second batch were not tested in human serum since the four dilutions in normal saline were negative.
Positive results: optical density indices of dilutions.
| Concentration of imipenem/cilastatin (mcg/mL) | In normal saline | In human serum | ||
|---|---|---|---|---|
| OD for Well 1 | OD for Well 2 | OD for Well 1 | OD for Well 2 | |
| 80 | 6.11 | 5.88 | 4.96 | 5.10 |
| 40 | 1.58 | 1.74 | 3.23 | 3.34 |
| 20 | 0.64 | 0.80 | 1.78 | 1.78 |
| 10 | 0.61 | 0.61 | 1.13 | 1.03 |
OD, optical density.
To the best of our knowledge, this is the first study evaluating the drug–laboratory interaction among the six generics of piperacillin/tazobactam available in the United States and other broad-spectrum antibiotics. All piperacillin/tazobactam brands produced negative results with the galactomannan antigen test in this study, which is consistent with the most recent data regarding this drug–laboratory interaction. Xavier et al.15 conducted an in vitro study in Brazil that investigated the drug–laboratory interaction with the brand and four generics of piperacillin/tazobactam available in Brazil at that time. When testing the concentrated medications (i.e., 45mg/mL), only one of the five brands of piperacillin/tazobactam produced a positive galactomannan antigen test, showing this drug–laboratory interaction to be manufacturer dependent.15 However, when testing the diluted medications (i.e., average peak serum concentration in the body, 300mcg/mL; one dilution above, 600mcg/mL; and two dilutions below, 150mcg/mL and 75mcg/mL), all concentrations produced a negative antigen test.15 These authors concluded that this drug–laboratory interaction between piperacillin/tazobactam and the galactomannan antigen test was no longer clinically relevant in Brazil.15 Similarly, Mikulska et al.16 conducted a study in Italy that investigated the drug–laboratory interaction with brand piperacillin/tazobactam (Tazocin™, Pfizer). When testing 90 randomly selected vials of piperacillin/tazobactam, none produced a positive antigen test.16 When testing patients’ serum, the antigen test was positive in slightly more serum samples from patients receiving piperacillin/tazobactam than those patients who did not, which was not found to be statistically significant (2.5% vs. 1.6%; p=0.18).16 These authors concluded that brand piperacillin/tazobactam no longer produces a false-positive result for the galactomannan antigen test; however, there is a concern with the multiple generics that are available.16 Based on our study, the drug–laboratory interaction with the six generics of piperacillin/tazobactam available in the United States revealed no positive results. Researchers have speculated that the reason for this drug–laboratory interaction no longer existing is secondary to changing manufacturing processes.8,17 Based on the package insert information, the brand name product was reformulated in 2005 from containing no excipients or preservatives to containing edetate disodium dehydrate and sodium citrate.18
This study did find that imipenem/cilastatin by Hospira Healthcare India Private Limited, a subsidiary of Hospira Inc., produced a positive antigen test. This finding is puzzling especially since imipenem/cilastatin is derived from a bacterium Streptomyces cattleya and, therefore, does not contain galactomannan and should not produce a positive galactomannan antigen test. More specifically, two batches of imipenem/cilastatin manufactured by Hospira were tested that produced conflicting results. In comparison of these two vials of imipenem/cilastatin, both batches had similar National Drug Code (NDC) numbers (i.e., same manufacturer, same medication, and different package size), different lot numbers, and different expiration dates. Batch 1 that produced a positive antigen test was manufactured by Hospira Healthcare India Private Limited (Chennai, India) while Batch 2 that tested negative was manufactured by Hospira, Inc. (Lake Forest, Illinois). The reason for these conflicting results remains unknown but may possibly be due to differing manufacturing processes between the two locations. Because positive results were seen with only one manufacturer, this observation may not be generalizable to imipenem/cilastatin as a whole.
Although this study reports in vitro data of limited scope, there are important clinical implications. Results suggest there is no longer a drug–laboratory interaction between piperacillin/tazobactam and the galactomannan antigen test, but there is a possible drug–laboratory interaction with imipenem/cilastatin (Hospira Healthcare India Private Limited). Knowing which medications produce a false-positive result for the galactomannan test is important since the results of this test may affect the treatment of patients resulting in unnecessary treatment, increased healthcare costs, risk of adverse effects, and drug–drug interactions.
Our study has several limitations. This study was an in vitro study, and not all in vitro data correlate with in vivo data. However, clinical samples were tested (i.e., four dilutions in serum) once the medication produced a positive result in normal saline. A single vial of each medication (i.e., one batch) was used in this study, which may not be generalizable to every manufacturer or lot. Lastly, only one in-date Platelia Aspergillus Ag kit was used for this study; thus, the number of tests that were run was limited.
In conclusion, this in vitro study suggests that imipenem/cilastatin by Hospira Healthcare India Private Limited produces a false-positive result for the galactomannan antigen test in at least some lots. Since the mechanism of this drug–laboratory interaction is unknown, this interaction should be further investigated with more in vitro studies testing additional batches of imipenem/cilastatin by Hospira Healthcare India Private Limited and in vivo studies testing patients’ serum who are receiving this medication. In addition, provider education will be necessary in order to ensure effective diagnoses and patient care in patients with suspected Aspergillus infections.
Conflicts of interestThe authors declare no conflicts of interest.