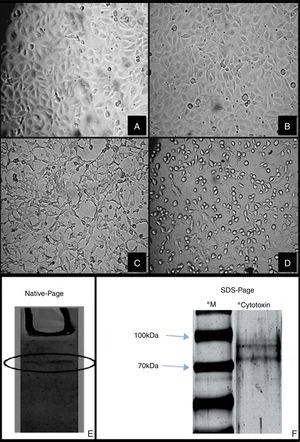
Culture supernatant of sepsis-associated Escherichia coli (SEPEC) isolated from patients with sepsis caused loss of intercellular junctions and elongation of human umbilical vein endothelial cells (HUVEC). The cytotoxic factor was purified from culture supernatant of SEPEC 15 (serogroup O153) by liquid chromatography process. PAGE (polyacrylamide gel electrophoresis) showed that the purified SEPEC cytotoxic factor had a molecular mass of ∼150kDa and consisted of at least two subunits. At the concentration of 1 CD50 (40μg/mL) did facilitate transcytosis through the HUVEC cells monolayer of SEPEC 15 as much as E. coli K12 within 30min without affecting cell viability. These results suggest that this cytotoxic factor, named as SPF (SEPEC's permeabilizing factor), may be an important SEPEC virulence factor that facilitates bacterial access to the bloodstream.
Bacterial sepsis is a condition in which bacteria invade the bloodstream and infect several organs. In these cases, the most frequently isolated microorganism from patient's blood is Escherichia coli.1,2 Sepsis-associated E. coli (SEPEC) belongs to the extra-intestinal pathogenic E. coli (ExPEC) group1,3,4 and is phylogenetically and epidemiologically different from both intestinal pathogenic E. coli and commensal E. coli, which are part of human microbiota.1,2 A variety of virulence factors related to human SEPEC include secreted toxins such as HlyA (α-hemolysin), Sat (secreted autotransporter toxin) and CNF-1 (cytotoxic necrotizing factor 1).2,5,6 These toxins can change the host cell shape and/or function, thereby contributing to the biological processes stimulated by the pathogen.7 Despite its medical importance, SEPEC is a poorly studied pathotype and it is still not known how these bacteria cross the endothelial barrier and gain access to the bloodstream.
In this study, we observed that culture supernatant of all SEPEC strains isolated from patients with sepsis (HC/UNICAMP, Campinas, SP, Brazil),3 were cytotoxic to human umbilical vein endothelial cells (HUVEC), causing loss of intercellular junctions, cellular elongation and death. In this way, we examined whether SEPEC's cytotoxic factor facilitates transcytosis of these strains in HUVEC monolayers, possibly indicating how SEPEC may reach the bloodstream during sepsis.
Sepsis-associated E. coli 15, serogroup O153,3 was selected among other SEPEC strains because this strain induced more intense cytotoxic effects on HUVEC cells (Fig. 1). This strain was grown in tryptic soy broth (TSB) for 5h at 37°C. The culture supernatant obtained by centrifugation was concentrated by a rotary evaporator (Marconi, SP, Brazil) and dialyzed (Spectrum, USA) against deionized water. This material was submitted to Mono-Q column chromatography (GE Healthcare), equilibrated with 0.04M Tris–HCl, pH 8.8, and eluted with a linear gradient of 0–1M NaCl in the same buffer. The elution profile was then monitored at 280nm and fractions (1mL/tube) were collected at a flow rate of 0.5mL/min.
Cytotoxicity of SEPEC cytotoxic factor on HUVEC. (A) HUVEC Cell Control, (B) HUVEC treated with E. coli K12 C600 culture supernatant, (C) HUVEC treated with chromatographic fractions (Superose 12 10/300GL), (D) HUVEC treated with SEPEC 15 culture supernatant. After treatment, all cells were incubated for 24h. Magnification for all images: 200×. Electrophoretic profile of purified cytotoxic factor in (E) native PAGE showing a single protein band and (F) SDS-PAGE showing two protein bands with molecular masses between 70 and 100kDa. **Active fractions obtained by gel filtration on Superose 12 10/300GL were run in both cases. *M – molecular mass markers.
The fractions with cytotoxic activity in HUVEC cells (Fig. 1C) were then applied on molecular exclusion chromatography column: Superdex 75 10/300GL column (GE Healthcare) followed by Superose 12 10/300GL column (GE Healthcare), equilibrated with 0.04M Tris–HCl, pH 8.8. The chromatographic fractions presenting biological effects that were obtained from all columns used in the purification processes were analyzed on native and sodium-dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The gels were stained with silver nitrate.8
Native PAGE of the chromatographic fractions from the last column chromatography showed only one band, indicating that the cytotoxic factor was electrophoretically purified. However, SDS-PAGE revealed the presence of at least two bands with molecular masses between 70 and 100kDa, which suggested that the SEPEC cytotoxic factor is a heterodimeric protein complex with a molecular mass of ∼150kDa (Fig. 1E and F).
The CD50 of purified SEPEC cytotoxic factor (40μg/mL) was previously described by Puerner and Borenfreund.9 The multiplicity of infection (MOI) of 20010 was determined as follows: The number of HUVEC cells was quantified using a Neubauer chamber and the number of CFU (colony forming units) by previous bacterial growth standardization by spectrophotometrical readings.
To assess cell monolayer permeability and transcytosis, 3-μm pore Transwell inserts for 24-well microplates (Thincerts™, Greiner Bio-One, Austria) were used. The insert membranes were coated with 200μL of collagen (Sigma) to give an equivalent coating of 10mg/cm2. After this, a suspension of 1×105 HUVEC in 350μL of minimal essential medium (MEM) was applied to the top of the collagen-covered insert. The bottom of the insert was filled with 1.2mL of MEM. The microplate with inserts was incubated at 37°C in a 5% CO2 atmosphere for 48h.
To evaluate changes in monolayer permeability, the cytotoxic factor (1 CD50) was applied to HUVEC monolayers grown above the insert (Thincerts™, Greiner Bio-One, Austria) and the microplates were incubated at 37°C in a 5% CO2 atmosphere (Shel Lab CO2 Incubator, OR, USA). The permeability of the monolayer was monitored by measuring the transendothelial electrical resistance (TEER) with voltohmmeter (Millicell – ERS EMD, Millipore, USA) after incubation with cytotoxin for 30, 60, 90 and 120min. Transwell inserts coated with collagen, but without cells, were used as zero controls. Inserts covered with collagen and cells, but without cytotoxic factor, were used as negative controls. The TEER negative control was considered as 100% since the monolayer was confluent and unaltered. The values recorded on the voltohmmeter were multiplied by the surface area of the insert (0.55cm2) to express the TEER units in Ω/cm2.
In the transcytosis assay, the protocol were as follows: the cytotoxic factor (1 CD50) and/or bacteria (at a multiplicity of infection – MOI of 200) were applied to HUVEC monolayers as follows: purified cytotoxic factor and septicemic E. coli (SEPEC 15); the cytotoxic factor and E. coli K12 C600; just septicemic E. coli (SEPEC 15) and E. coli K12 C600. The microplates were incubated at 37°C in a 5% CO2 atmosphere (Shel Lab CO2 Incubator, OR, USA). After 30, 60, 90, and 120min of incubation, 10μL of sample was collected from the lower chamber of the insert and plated on MacConkey agar and incubated at 37°C for 24h. After that the colony forming units (CFU) were counted.
The permeability evaluation and the transcytosis assays were done in triplicates and the results were analyzed by the software GraphPad Prism 5 with the one way ANOVA method with Tukeys and Dunnetts Tests, respectively.
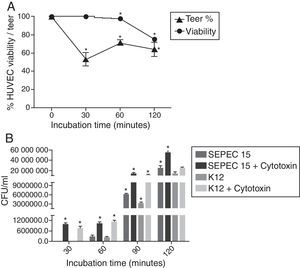
The TEER of HUVEC monolayers was reduced by nearly 50% after a 30min incubation with the cytotoxic factor, indicating an increase in monolayer permeability (p<0.0001). The permeability stabilized from 60minutes onwards, with a TEER that was 62–72% of that seen in confluent monolayers not exposed to cytotoxin (Fig. 2A). Incubation with the cytotoxic factor enhanced the translocation of bacteria across the HUVEC monolayer since a greater number of bacteria underwent transcytosis in the presence of this factor, than in cells not exposed to the cytotoxin (p<0.0001).
(A) Transendothelial electrical resistance (TEER) of HUVEC monolayers after incubation with the cytotoxic factor for 240min. The TEER was expressed as a percentage of the zero control. (B) Transcytosis of SEPEC15 and E. coli K12 C600 in the absence and in the presence of purified cytotoxic factor. *p<0.0001.
The SEPEC cytotoxic factor causes elongation and loss of intercellular junctions in Vero (African green monkey kidney epithelial) cells (data not shown), same as observed in HUVEC cells (Fig. 1). Guyer et al.11,12 observed that SAT (autotransporter toxin), a protein of 142kDa, produced by UPEC (uropathogenic E. coli), causes a cytotoxic effect in Vero cells. However, the SEPEC 15 does not harbor the Sat encoding-gene.3
Furthermore, the culture supernatant of SEPEC 15 obtained before 4h or after 6h of culture showed negligible or no cytotoxicity in HUVEC cells (data not shown). Based on the growth pattern observed for this strain, we suggest that the cytotoxic factor may be secreted mainly at the exponential growth phase and undergoes degradation in the stationary phase, as observed for some proteins produced by Chlamydomonas reinhardtii.13
The measurement of the transendothelial electrical resistance (TEER) showed that the SEPEC cytotoxic factor, which caused elongation and loss of intercellular junctions in HUVEC, also increased the cell monolayer permeability by ∼46% within 30min. Sandoval et al.14 reported that HUVEC monolayer permeability could be increased by altering the function of the intercellular junction proteins VE-cadherins through the activation of PKCα (a protein kinase C isoform). Similarly, Sukuruman and Prasadarao15 observed increased permeability of human brain microvascular endothelial cell (HBMEC) monolayers following the activation of PKCα, but with a loss of functional intercellular junction proteins. The activation of PKCα in HBMEC involves the interaction of OmpA (outer membrane protein A) produced by E. coli K1 with the Ecgp (endothelial cell glycoprotein) receptor. This interaction does not occur in systemic endothelial cells such as HUVEC. Thus, we suggest that the permeability observed in HUVEC monolayers resulted from the interaction between the secreted cytotoxin and HUVEC-specific receptors, which then activate the mechanism described by Sandoval et al.,14 and Sukuruman and Prasadarao.15 Nevertheless, further studies are needed to confirm this hypothesis.
In addition to increasing the HUVEC monolayer permeability, the purified SEPEC cytotoxic factor facilitated the translocation of bacteria across the monolayer (transcytosis). This transcytosis occurred within 30min after infection, without affecting the viability of the monolayer. Approximately 1h after infection, when >95% of the HUVEC were still viable, approximately 0.05% (∼1×104CFU) of the bacteria applied were recovered in the lower chamber of the insert.
For the transcytosis assay, bacterial suspensions (MOI=200) of SEPEC 15 or E. coli K12 C600, were applied to the Transwell insert (Thincerts™, Greiner Bio-One, Austria) in the addition of 1 CD50 of purified SEPEC cytotoxic factor. The transcytosis was observed at 30min of infection. However, when the cytotoxin was not added, bacterial translocation was observed after 1h of infection. This effect was probably due to the cytotoxicity of the protein on HUVEC cells. At all post-infection intervals, a greater number of SEPEC 15 passed through the HUVEC monolayer when compared with the non-pathogenic strain. This observation could be explained by the ability of SEPEC 15 to produce and secrete this cytotoxic factor, unlike K-12 C600. In both cases, at its CD50 concentration, the purified cytotoxin enhanced translocation, resulting in a more than two-fold increase in the number of bacteria crossing the monolayer without the aid of the cytotoxin.
Thomas et al.16 showed that the Treponema pallidum, which causes syphilis, increased the permeability of HUVEC monolayers and translocated through the monolayer. These authors16 observed that within six hours of infection, 7.2% of the bacteria passed through the monolayer, even with a cell viability >99%. By using electron microscopy, they showed that during transcytosis T. pallidum was located between the cells and not within them, suggesting that bacterial translocation involved a paracellular pathway. In contrast, Comstock and Thomas17 demonstrated that the translocation of Borrelia burgdorferi through HUVEC monolayers occurred without alterations in the monolayer permeability. These authors17 found that four hours after infection, 9.6% of the bacteria had translocated and 98% of the cells were still viable. The bacteria were observed within the cells, indicating that the translocation of B. burgdorferi involved an intracellular pathway. Based on these results16,17 and since, in the present study, the translocation of E. coli was accompanied by an increase in monolayer permeability (seen as a decrease in the TEER), we suggest that the cytotoxic factor secreted by SEPEC 15 facilitates its passage through the endothelial barrier by a paracellular pathway following alterations in the functional intactness of the intercellular junctions.
Based on the results of Guyer et al.11, Kaper et al.18 suggested that the Sat cytotoxin may damage venous capillaries, which are often only one cell thick. Thus, the SEPEC cytotoxic factor, described here, could facilitate the passage of bacteria through the endothelial barrier in the early stages of sepsis in a similar way to Sat cytotoxin. However, no studies have yet shown that Sat or any other cytotoxin secreted by E. coli can change the permeability of endothelial cell monolayers and/or facilitate bacterial transcytosis. Nevertheless, the transcytosis of E. coli and a change in endothelial cell monolayer permeability was described by Sukuruman and Prasadarao in 2003.15 These authors showed that E. coli K1 strain RS 218 (serotype O18:K1:H7), which is associated with neonatal meningitis, crosses HBMEC (human brain microvascular endothelial cells) monolayers with the help of the outer membrane protein OmpA. Other studies have reported changes in the TEER and the translocation of bacteria through endothelial cell monolayers using different interaction bacteria/endothelial cell models.10,19–21 However, the present study is the first to report changes in permeability in HUVEC monolayers caused by a cytotoxin secreted by SEPEC. Due to the biological activity observed, we named that cytotoxic factor as SEPEC Permeabilizing Factor (SPF). Furthermore, our data suggest that SPF takes part in early stages of sepsis, by assisting bacterial translocation across the endothelial barrier and facilitating access to the bloodstream. However, further studies are needed to establish the mechanisms involved in the translocation of human SEPEC.
Conflicts of interestThe authors declare no conflicts of interest.
The authors wish to thank Ana Stella Menegon Degrossoli for technical assistance and Stephen Hyslop for helping with the English language review. We are also grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) for providing financial support.