Infections caused by emerging Cryptococcus non-neoformans species are being reported with increasingly frequency. Here, we present a case of fungaemia by Cryptococcus laurentii in a woman receiving aggressive immunosuppressive therapy for cervical neoplasia. Three venous blood samples were aseptically collected on consecutive days and C. laurentii was isolated and identified through phenotypic and molecular methods. After central venous catheter removal and appropriate antifungal therapy, the patient showed significant improvement and blood culture became negative. Thus, patients following immunosuppressive therapies and using invasive medical devices are at risk of C. laurentii blood infections.
Cervical cancer is an important malignancy condition worldwide, with a significant mortality rate in developing countries, which corresponds to annual incidence of approximately 132,000 cases. According to the Brazilian National Cancer Institute (INCA), there are 18,430 newly reported cases per year and this cancer is the fourth leading cause of death in female population represented by 4800 cases with fatal outcome.
The therapies of choice and the underlying conditions of cancer had led to an increasing number of severe opportunistic fungal infections such as cryptococcosis.1 Commonly, it is caused by Cryptococcus neoformans and Cryptococcus gattii, with recent reports by non-neoformans Cryptococcus species as C. laurentii which is a basidiomycetous encapsulated yeast, also found in the droppings and cloacal samples of pigeons.2
Cryptococcus laurentii generally cause superficial to deep seated infections in immunosuppressed patients. The fungal infection may be acquired by inhalation leading to asymptomatic pulmonary infection or through the use of contaminated invasive medical devices. Also, other risk factors include: prior steroid and immunosuppressant exposure, azoles prophylaxis, low CD4 count, and neutropenia.2
Case reportThe procedures described herein are in accordance with ethical standards of the Ethics Committee on researches involving humans from the Universidade Federal de Pernambuco/Brazil.
A 42-year-old Brazilian woman was admitted in December 2012 to the Hospital das Clínicas, a public tertiary hospital located in Recife, Brazil. She had had fever for one week and diffuse abdominal pain and discomfort during intercourse. A complete blood count revealed no abnormalities and blood and urine cultures were also negative for bacteria and fungi in this occasion. Colposcopy procedures exhibited an abnormal area in the cervix, and the Papanicolaou test showed abnormal cells. Cervical colposcopic biopsies, endocervical curettage and cone biopsy diagnosed cervical intraepithelial neoplasia (CIN) grade 3 (squamous cell carcinoma). Chemotherapy and radiotherapy elicited good responses and ultimately a full clinical recovery. Subsequently the patient was discharged home.
However, one year thereafter the patient was hospitalized with diabetes mellitus, abdominal distension, appetite loss, hypoalbuminemia, and intestinal sub occlusion, characteristic of an actinic stenosis. Bowel resection and jejunostomy were required to correct the intestinal stenosis, with concomitant administration of parenteral nutrition and antibiotic therapy.
In January 2014, due to adhesions between bowel loops associated with stenosis and presence of mucous fistula, another corrective enterectomy was performed which revealed the presence of fecal content, intestinal evisceration and aponeurotic dehiscence. Postoperatively, a surgical wound infection caused by Pseudomonas aeruginosa, Citrobacter sp. and Acinetobacter sp., aggravated by sepsis and a generally worsening clinical condition required long-term antibiotic therapy involving intravenous vancomycin, followed by imipenem and metronidazole. However, three months later she was readmitted to the intensive care unit presenting fever episodes, severe abdominal pain, weakness and respiratory distress. Laboratory investigations showed elevated urea (207mg/dl) and creatinine (4.0mg/dl) levels, leukopenia (WBC 3.60×103/μl), low hemoglobin (8.0g/dl) and platelets (8500/μl) levels.
Duplicate venous blood samples were aseptically collected into VACUTAINER® tubes using EDTA anticoagulant and blood cultures (BACTEC 860 system; Becton Dickinson, Inc., Sparks, MD) on three consecutive days. Samples were processed by standard methods (direct examination and culture) for mycological diagnosis at the Medical Mycology Laboratory, Federal University of Pernambuco. Direct examination was performed with and without India ink. Collected material was cultured on Sabouraud Dextrose Agar medium (Difco Laboratories) supplemented with chloramphenicol, incubated at 25°C and 37°C in an aerobic atmosphere for 10 days. The taxonomic identification was carried out with pure cultures and also using molecular techniques by comparing D1/D2 sequences of the isolated yeast strains with the type strain CBS 139/Genbank AF075469.3
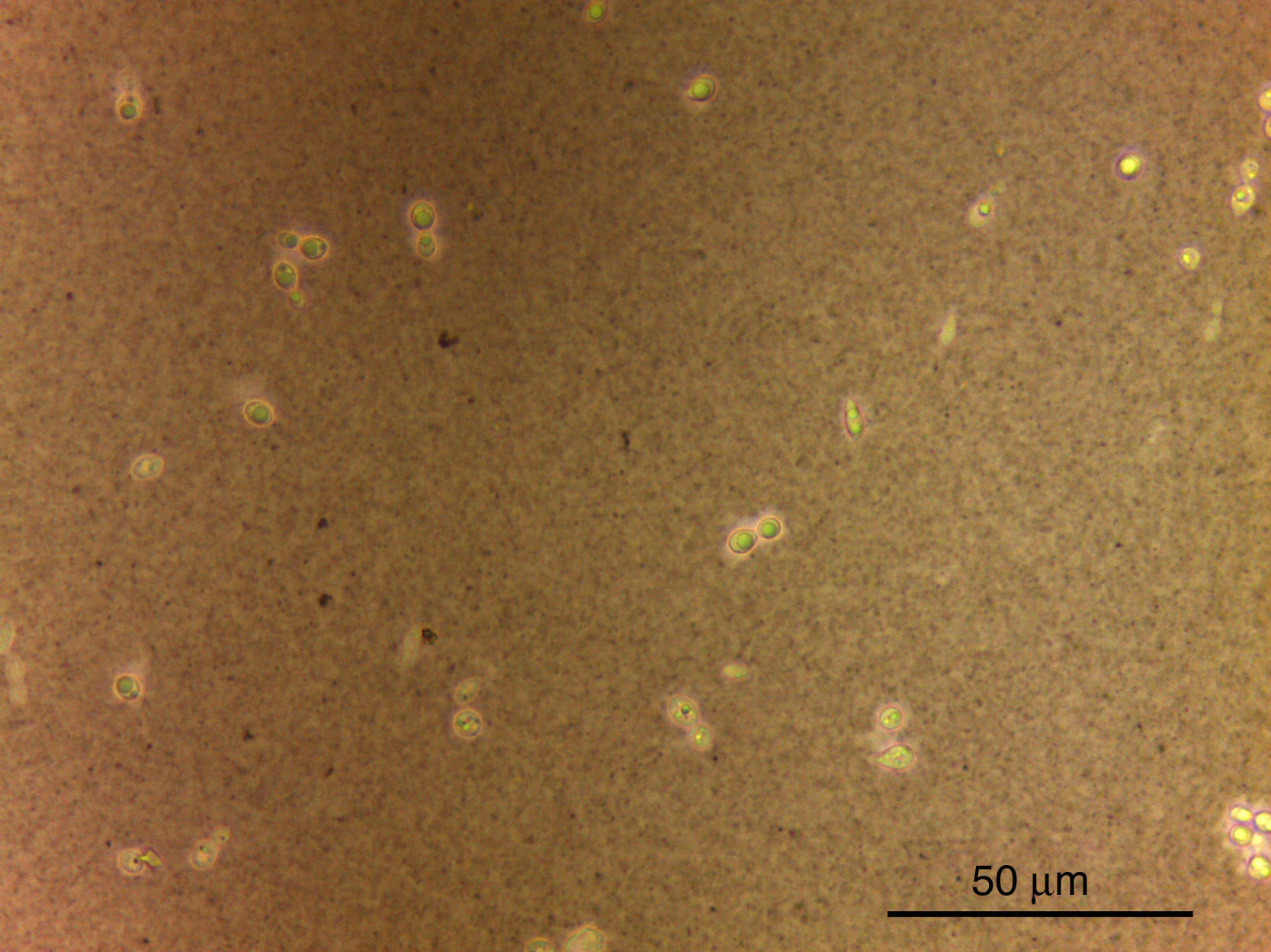
Budding capsulated yeast cells were visualized directly in wet films with contrast microscopy (India ink) and all blood cultures were positive after five days of growth (Fig. 1). Macroscopically, 50–100 white-cream colonies grew, with mucoid to butyrous consistence, which darkened with age. The reverse was colorless. Microscopically, contrasted budding encapsulated spherical and ellipsoidal yeast cells of approximately 5μm in diameter were observed, which was morphologically consistent with their identification as Cryptococcus species.
Nonfermentative colonies, absence of KNO3 utilization, caffeic acid negative, and urease positive reactions were observed through standard identification techniques. The assimilation profile showed utilization of lactose, d-glucuronate, d-gluconate, and melibiose, which indicates C. laurentii as the agent of the bloodstream infection. The identification of the microorganism was also confirmed using molecular techniques.
In addition, bronchoalveolar lavage and cerebrospinal fluid were analyzed through direct exam, culture, and cryptococcal latex agglutination. However, no fungi were detected.
The minimal inhibitory concentrations (MICs) of the etiological agent isolated from blood samples against fluconazole and amphotericin B were determined using the broth micro-dilution method according to the M27-A3 document from the Clinical and Laboratories Standards Institute-CLSI.4 The isolates were sensitive to amphotericin B (1.0μg/ml) and fluconazole (4μg/ml).
Due to persistent elevated levels of urea and creatinine, fluconazole was the drug of choice and the patient was treated intravenously (400mg) for 22 weeks. After central venous catheter removal she showed significant improvement and was discharged home.
DiscussionIn the case herein described a woman with an underlying malignancy was severely immunocompromised due to the immunosuppressive effects of radio and chemotherapy cycles and due to her long-standing diabetic status. Additionally, the patient had a predisposition due to the use of multiple catheters and invasive medical devices, which were considered the possible source of fungaemia, although catheters cultures were not evaluated during hospitalization.
Cryptococcus commonly invades the human body after inhalation, through the alimentary tract or injured skin. From the portal of entry, the encapsulated yeast cells are easily transported via bloodstream to other parts of the body. The presence of invasive devices is considered a significant risk factor associated with C. laurentii infections.2,5 In our case, the catheter tip was only removed during the antifungal treatment and has not been sent for microbiological analysis.
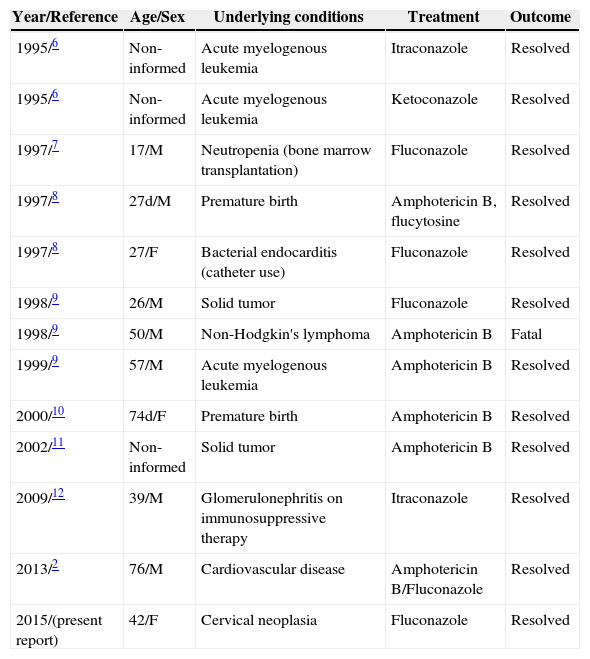
Furthermore, what makes this case relevant is the fact that fungaemia was caused by a rather uncommon microorganism, C. laurentii, for which there is still a paucity of data in the literature. For instance, there are few published cases for both superficial and deep-seated infections, including fungaemia (Table 1).
Reported cases of Cryptococcus laurentii fungaemia in humans.
| Year/Reference | Age/Sex | Underlying conditions | Treatment | Outcome |
|---|---|---|---|---|
| 1995/6 | Non-informed | Acute myelogenous leukemia | Itraconazole | Resolved |
| 1995/6 | Non-informed | Acute myelogenous leukemia | Ketoconazole | Resolved |
| 1997/7 | 17/M | Neutropenia (bone marrow transplantation) | Fluconazole | Resolved |
| 1997/8 | 27d/M | Premature birth | Amphotericin B, flucytosine | Resolved |
| 1997/8 | 27/F | Bacterial endocarditis (catheter use) | Fluconazole | Resolved |
| 1998/9 | 26/M | Solid tumor | Fluconazole | Resolved |
| 1998/9 | 50/M | Non-Hodgkin's lymphoma | Amphotericin B | Fatal |
| 1999/9 | 57/M | Acute myelogenous leukemia | Amphotericin B | Resolved |
| 2000/10 | 74d/F | Premature birth | Amphotericin B | Resolved |
| 2002/11 | Non-informed | Solid tumor | Amphotericin B | Resolved |
| 2009/12 | 39/M | Glomerulonephritis on immunosuppressive therapy | Itraconazole | Resolved |
| 2013/2 | 76/M | Cardiovascular disease | Amphotericin B/Fluconazole | Resolved |
| 2015/(present report) | 42/F | Cervical neoplasia | Fluconazole | Resolved |
M, male; F, female; d, days.
A review of the medical literature indicated that this report represents the first isolation of C. laurentii from the blood of a patient with cervical neoplasia under radio and chemotherapy. A systematic review by Khawcharoenporn et al.5 showed that non-neoformans and non-gattii cryptococcal infections occur sporadically, with C. neoformans and Cryptococcus albidus accounting for 80% of the reported cases.
Due to the rarity of cases involving C. laurentii, a standard treatment has not yet been established. Commonly, amphotericin B with flucytosine is recommended. However, azole derivatives have been used successfully in some cases5 as verified in the present case.
Susceptible patients, especially those at risk due to immunosuppressive therapies, might inadvertently come into contact with such species and develop nosocomial infections. Interestingly, it appears that C. laurentii, a non-C. neoformans species, has managed to expand beyond its normal environmental niche to become a recognized human pathogen.
Conflicts of interestThe authors declare no conflicts of interest.
Authors’ contributionRPN and DPCM conceived the manuscript, participated in the collection of clinical data, molecular tests, and manuscript writing; RGLN and VKAS participated in the collection of clinical data and manuscript revision; MCL and FAGS participated in the Laboratorial technical support and molecular tests. All authors read and approved the final manuscript.
The authors would like to thank Department of Mycology from Universidade Federal de Pernambuco, for their technical support and kind assistance. This work was supported by the Coordination of Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq)/Brazil.