Due to the emergence of multi-drug resistant bacteria, and the evident limitation in therapeutic options, alternatives to combat bacterial infections have been sought. One of these is phage therapy, which is the use of bacterial viruses to kill pathogenic bacteria responsible for the infection. These viruses called bacteriophages are very abundant organisms in the world and are harmless to humans. There are several advantages in using phage therapy, especially against multi-drug resistant pathogens, which tend to be dominated by individual strains. The advantages include fewer collateral effects such as lower disturbance of gut microbiota and less antimicrobials consumption, which itself leads to reducing antibiotic resistance rates. Unfortunately, few clinical studies have been initiated in Brazil and this area is little explored in our country. This manuscript describes clinical evidence of successful phage utilization on pathogens considered a threat in Brazil, highlighting the benefits of a possible phage utilization as an important tool to combat antimicrobial resistance in our country.
Antimicrobial resistance in Brazil has been a frequent topic of discussion over the last few years. Currently, the country is experiencing a scenario where resistance to antimicrobial agents is emerging so quickly, that many people are claiming that the antibiotic era is coming to an end. Although new combinations of β-lactams and β-lactams inhibitors are being introduced into clinical use, multi-drug and pan-drug resistant isolates have been characterized around the world calling into question how much one should be held hostage to the sole use of antimicrobial agents in treating infections. Since there are numerous review manuscripts addressing phage therapy, the purpose of this review is to provide a general overview about phage utilization, highlighting the Brazilian scenario with includes the main pathogens or lineages which need concentrated efforts and the Brazilian investigations conducted on this topic. In this review, supportive papers will be indicated to improve the understanding about phage utilization.
Antimicrobial resistance of Gram-negative bacilli as a public health threatAntimicrobial resistance has been considered a threat to public health in different parts of the world, including middle and low-income countries such as Brazil.1 The increasing emergence of multi-drug resistant (MDR) bacteria leads to poor clinical outcomes for nosocomial patients and increases the costs associated with hospitalization.2 According to the Brazilian Health Regulatory Agency (ANVISA) an increase in carbapenem resistance rates among Gram-negative bacilli has been observed in the last few years, especially in pathogens like Acinetobacter spp., Klebsiella pneumoniae, and Pseudomonas aeruginosa recovered from blood stream infections (BSI) in 2016 (85%, 46.8%, and 42.9%, respectively).3 Although national reports only focus on cephalosporins, carbapenems, and more recently polymyxin, studies have demonstrated the presence of bacterial isolates with multi-drug resistance or extreme drug resistance (XDR) in different Brazilian regions.4–8
Some antibiotic resistant bacterial clones are endemic in Brazil, leading to high resistance levels at Brazilian hospitals. Among them, KPC-2-producing Klebsiella pneumoniae (CC258, ST258 and ST11), SPM-1-producing Pseudomonas aeruginosa (ST277), and carbapenem-hydrolyzing class D carbapenemase-producing in Acinetobacter baumannnii (ST79) have gained clinical importance in recent decades.9 For the last 5–6 years, especially due the emergence of KPC-2 producing K. pneumoniae, polymyxin has been largely used to treat infections caused by these Brazilian endemic clones. On the other hand, emergence of polymyxin resistance, mainly among K. pneumoniae and A. baumannii isolates, has been detected, limiting even more the therapeutic choices for treating such infections.10,11
For this reason, researchers from many nations worldwide are trying to combat infections caused by MDR pathogens by using alternative methodologies. Phage therapy is one of these alternatives to traditional antibiotic therapy and has been used with great effectiveness in some countries.12
Bacteriophages: viruses responsible for bacterial biological controlBacteriophages, also called phages, are viruses with the ability to infect and kill bacteria and are the most abundant organisms on Earth.13 They are found in different environments, and frequently detected in water, sewage, soil, food, clinical samples, and others.14 Phages usually infect and kill only a single bacterial strain or lineage thus not able to infect all types of bacteria. This specific nature is very positive in the clinical setting, since only pathogenic bacteria would be targeted, infected and killed, preserving commensal harmless and perhaps beneficial bacteria.13,14 The bacteriophage infection cycle can be complex.15 After infecting the bacteria, the phages can show a lytic or lysogenic cycle. Briefly, in the lytic cycle, the most important cycle for the phage therapy, the virus infects the bacteria cell, multiplies itself using the cell machinery, and after a period of time (typically 10–20min) kills the bacteria by a lytic phenomenon. After lysis, the countless new viral particles are able to infect new bacterial cells.13,16 In contrast, in the lysogenic cycle, after infecting the cell viral genetic material is incorporated on the bacteria genome, staying there until exogenous stimuli activate its lytic cycle.13,16 It is important to notice that for phage therapy, lysogenic phages are not useful since the necessary type of stimulus is oftentimes unknown, or requires extreme conditions such as pH, UV light, or low temperatures.17,18
When bacteriophage was first discovered, their clinical potential was quickly appreciated. However, initial studies were fraught with problems and often not well-designed yielding unsatisfactory results.19 This fact combined with the discovery of antibiotics led to a declining interest in the use of bacteriophage as a useful therapy in Western medicine. It is important to notice that although the phage therapy was not cogitated during some years in several parts of the world, it was largely used in countries of the former Soviet Union, as Georgia and Poland.20 However, since the expansion of antibiotic resistance in Gram negatives in the 90s,19 interest in phage therapy has increased substantially with many papers being published. These new studies were well conducted, and satisfactory results started to be obtained, stimulating more and more interest in clinical bacteriophage utilization.13,14 In the last years, distinct phages have been characterized with great potential to combat several infections caused by MDR pathogens.
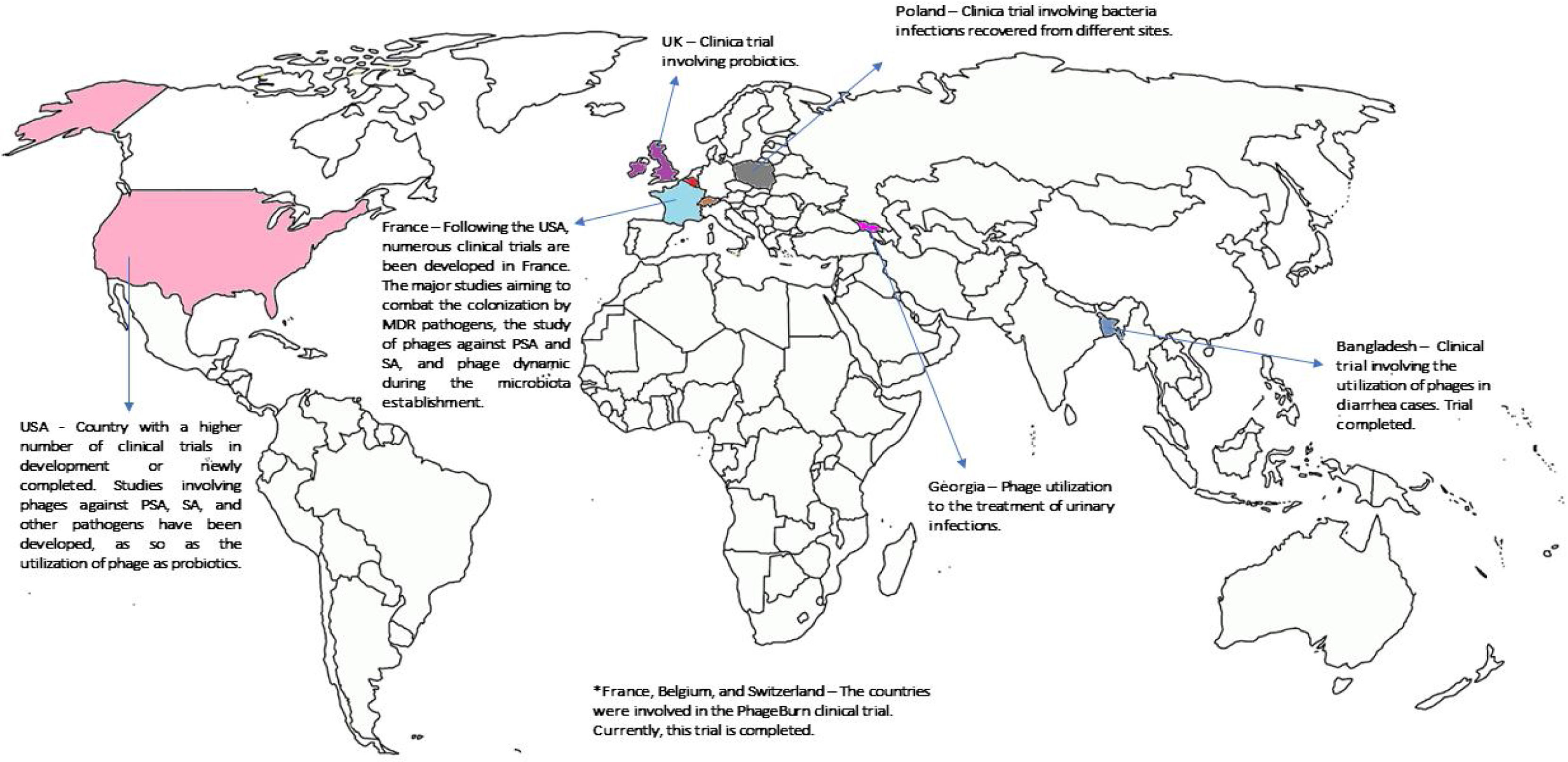
Current clinical studies supporting the phages utilizationAs discussed before, phage therapy is based on the use of lytic phages to kill infectious bacteria whilst leaving human cells unaffected and with a minor impact on human commensal bacteria.12 Currently, phages are largely used in the food industry, being considered by FDA as Generally Regarded as Safe (GRAS).21 One of the biggest achievements in the phage field was the approval of the first product based on phage (ListShield™) in 2006, being registered for use as an organic food additive in Europe, Australia and New Zealand.21 Although phage use is frequent by food companies, this review will emphasize the utilization of these microorganisms in clinical medicine. Based on that, many clinical trials are in progress (Fig. 1) stimulated by the good results obtained in other fields. Here, we will summarize some clinical trials against Gram-negative pathogens considered a threat in Brazil. It is important to notice that this review will focus on a selection of trials and clinical reports considered by the authors as relevant to support a future clinical utilization of phages in Brazil. To better understanding the studies involving human and animals related to phage utilization in emergent pathogens, read Mulani et al.22
World distribution of clinical trials in humans being conducted or newly concluded. It is possible to note that just few countries have invested in clinical trials supporting phage utilization in humans. To have more information about the clinical trials, see the website https://clinicaltrials.gov/.
Phages against MDR A. baumannii isolates have mostly been described in the last couple of years. Turner et al.23 performed a genomic analysis of 37 phages previously deposited in GenBank and recovered from A. baumannii isolates. So far, none of the phages described and characterized were recovered from South American samples.23 Despite, a large number of phages that have been fully characterized, only two clinical trials in humans have been reported until now. In the first one, a 68-year old diabetic patient was diagnosed with a chronic pancreatic pseudocyst aggravated by an MDR A. baumannii infection during his hospitalization. Due to limitation of therapeutic options (susceptible just to minocycline), a phage cocktail was introduced together with antibiotics. Phage therapy was maintained for more than four months, with a complete recovery of the patient's condition. During phage therapy, the bacterial isolate recovered causing the infection developed resistance to some phages used in the cocktail, but in return showed increasing antibiotics susceptibility. The phage resistance was circumvented adding new phages to the cocktail with activity against MDR A. baumannii. No collateral effects due to phage utilization were observed in the patient.24
The second clinical test involved A. baumannii isolates, in a 77-year old male patient, admitted to a hospital in San Diego, USA with a brain injury. The patient was submitted to craniectomy, followed by infection after the surgical procedure. Intraoperative material cultured giving growth to an MDR A. baumannii isolate only susceptible to colistin (some cultures showed A. baumannii ColS and others ColR isolates). An attempt to cure the infection with drug combinations including non-usual antimicrobial agents used to treat A. baumannii infections, such as rifampin+colistin, azithromycin+colistin, and chloramphenicol+colistin was unsuccessful, leaving phage therapy as one of the few alternatives for treating the patient. Five phages were detected with activity against the isolate recovered from this patient. The phage was administered intravenously after 12 days of hospitalization. It is not clear how many different phages were used in the cocktail, but an improvement in his health condition was noted. Twelve days thereafter the patient was only treated with phages because colistin was suspended on day 13 due to kidney injury. The patient received eight days of phage therapy. However, before administration of the second phage cocktail, his family opted to withdraw treatment and phage therapy was discontinued on day 19, and the patient died on day 20. Nevertheless, it is interesting to note that while the phages were being administered the patient's health condition was maintained with some clinical improvement.25
Pseudomonas aeruginosaAlthough P. aeruginosa infections were treated with bacteriophages more than 50 years ago,26 this therapy has recently reemerged as an attractive alternative treatment.27P. aeruginosa is an opportunist pathogen, historically associated with severe nosocomial infections and with a strong link with cystic fibrosis and burn infections. It often displays an MDR phenotype and numerous studies involving phages against P. aeruginosa have been reported.27,28 Besides the capacity to accumulate distinct resistance mechanisms, this microorganism is also able to produce a high level of biofilm, especially on inanimate surfaces. Therefore, phages active against the biofilm and the planktonic cultures are also important for treating P. aeruginosa infections.28
Ten years ago, a clinical trial involving bacteriophage therapy for P. aeruginosa infections was published demonstrating that bacteriophage therapy against P. aeruginosa infections was safe and clinically effective. Twenty-four patients with external otitis caused by P. aeruginosa strains were divided into two (n=12 each) treatment groups. Group 1 was treated with a cocktail containing six phages with activity against P. aeruginosa (105 PFU of each phage diluted in a solution) while the group 2 was treated with placebo. To be part of the study, the patients’ bacteria were submitted to microbiological tests to check if they were susceptible to at least to one phage contained on the cocktail. The phage therapy was effective in combating the infection and improving the ear health of the patients. In addition, it was possible to detect how long the phages remained in the region where the solution had been applied. According to the authors, when the target bacterial is reduced or completely eliminated, the phage replication ceases leading to phage elimination. No signal of toxicity or collateral effects were reported in this study, highlighting the safety of this phage therapy.29
In Europe, a platform named PhageBurn represents one of the biggest research studies in the phage field against P. aeruginosa and Escherichia coli from burn infections, and involved countries such as Belgium, France, and Switzerland (http://www.phagoburn.eu). Recently, a double-blind randomized controlled trial was published using phages against P. aeruginosa responsible for burn infections. In this study two groups of patients either received a phage cocktail (n=12/13) containing 12 phages active against P. aeruginosa isolates, or a traditional treatment (n=13) using a standard of care for patients with burn infections (1% sulfadiazine silver emulsion cream). The phage therapy was given to patients through a topical cream containing the phages. Unfortunately, some unexpected events compromised the study, and the trial was stopped one year and a half after its beginning. After the cream was manufactured, the phage concentration decreased from 1×106 PFU to 1×102 PFU, (less than initially expected). This resulted in inefficacy of PP1131 (a phage cocktail) due to the extensive time taken to decrease the bacterial density on the wound infection. However, it is important to notice that although a lower phage concentration was used, the bacterial burden in burn wounds was decreased, showing that phages were in fact active, but inferior to the standard of care treatment.30
Klebsiella pneumoniaeSeveral studies have focused or suggested alternatives to the treatment of infections caused by K. pneumoniae.12,31 Thus, several different types of phages have been discovered in the last few years. Although it represents an important advance in the field, the number of clinical trials using phages against K. pneumoniae clones are insufficient to determine whether phage therapy is a viable option for treating Klebsiella infections in humans.32–34
Recently, Corbellino et al.35 demonstrated the utilization of a custom-made bacteriophage lytic preparation to gut decolonization of a patient colonized by KPC-3-producing K. pneumoniae. A 57-year old patient diagnosed with Crohn's disease had multiple sites of colonization by an MDR K. pneumoniae belonging to ST307. The isolate was susceptible to ceftazidime-avibactam, which was successfully with reversion of all infection signs, but with later remission. Probably, the repeated cases of infections (UTI and BSI) by the same pathogen could be attributed to the patient colonization, evidenced by swab cultures. Five isolates recovered from the patient were sent to Georgi Eliava Institute of Bacteriophages in Tbilisi, Georgia for custom of phage preparation. Interestingly, after three weeks of phage use by oral and intra-rectal routes and no adverse effects, the patient did had no urine, blood, or swab positive cultures with carbapenem-resistance. Almost one year after the phage utilization, the patient had other cases of BSI and UTI by K. pneumoniae; however, the recovered isolates belonged to other STs (ST1109 and ST247), susceptible to most antimicrobial agents, and not related to the KPC-3-producing K. pneumoniae only susceptible to ceftazidime-avibactam frequently detected before.35
Kumari et al.36 compared phage therapy and its respective protection in a wound burn model. For that study, three groups of mice were created: the first consisted of untreated animals, the second were treated with a hydrogel containing the phages against a K. pneumoniae isolate, while the third group was treated with gentamicin plus silver nitrate. The results demonstrated that a single dose of phages resulted in decreased mortality of the group treated with phages when compared with the other groups.
Role of phages in infection control: a good way to combat MDR pathogensPhages are recognized as useful tools to combat infections caused by many types of microorganisms, including MDR-pathogens.16 Some studies have also used phages on hand sanitizer solution aiming to improve hand hygiene and avoid the spread of MDR pathogens.37 Other authors have demonstrated the capacity of decolonization of specific isolates by phage action.38,39 Chhiber et al.39 have demonstrated that phages in association with muropirocin were able to effectively eradicate the colonizing MRSA population from the nares of BALB/c mice. More importantly, administration of phage MR-10 combined with muropirocin reduced the frequency of emergence of spontaneous muropirocin resistant isolates at negligible levels. In addition, phages also could be used for effective removal of hospital pathogens from hard surfaces at hospital settings, especially when used in combination with detergents.40 In a two-phase prospective intervention study performed in Taiwan, the use of an aerosol formulation containing bacteriophage in the ICU led to an important reduction of new acquisition carbapenem resistant A. baumanii infection from 8.57 per 1000 patients-day to 5.11 per 1000 patients-day (p=0.0029). Moreover, a decrease in carbapenem-resistance was observed (87.76% to 46.07%) in the ICUs.41 These actions could reduce the number of infections and, indirectly, reduce antibiotic usage for treating severe infections.41
It is well known that the use of large amounts of antimicrobial agents is responsible for the selection of resistance clones, contributing to high rates of antimicrobial resistance that are frequently reported in Brazil.3 Another advantage that supports phage utilization in developing countries is the low cost associated with phage isolation and administration.14 The process of discovery and production of a new antimicrobial agent is expensive in terms of time and resources. In contrast, phages could be easily identified in water environments, especially in sewage, through a simple process of isolation and purification.14 In Belgium, for instance, phages can be used as a magistral formula, manipulated by a pharmacist, following a series of criteria which determine the phage safety.42,43 Regarding administration, while repeated doses of antibiotics need to be administrated periodically to achieve the pharmacokinetic and pharmacodynamic parameters, small doses of phages are enough since the number of viral particles will expand after the cell infection.20 In countries with high social inequalities and limited resources to invest in public health, low cost and effective alternatives could be essential to tackle the high rates of deaths caused every year by MDR-pathogens in nosocomial environments.
In addition, phages could be used in combination with antimicrobial agents increasing therapeutic efficacy and decreasing antimicrobial resistance development during therapy.44,45 Another important advantage related to phage utilization is the capacity to restore antimicrobial sensitivity.46 A study performed with a phage named OMKO1 revealed the ability of this phage to interact with the outer membrane proteins of MexAB and MexXY efflux systems in P. aeruginosa isolates. In a strategy to survive viral attack, the cell seems to develop a mechanism by which several mutations in the outer membrane proteins are selected during bacteria passage, decreasing virus-bacteria binding. However, this modification on the outer membrane protein sequence decreased the capacity of bacteria to extrude the antibiotics, causing a drop in the MICs.46
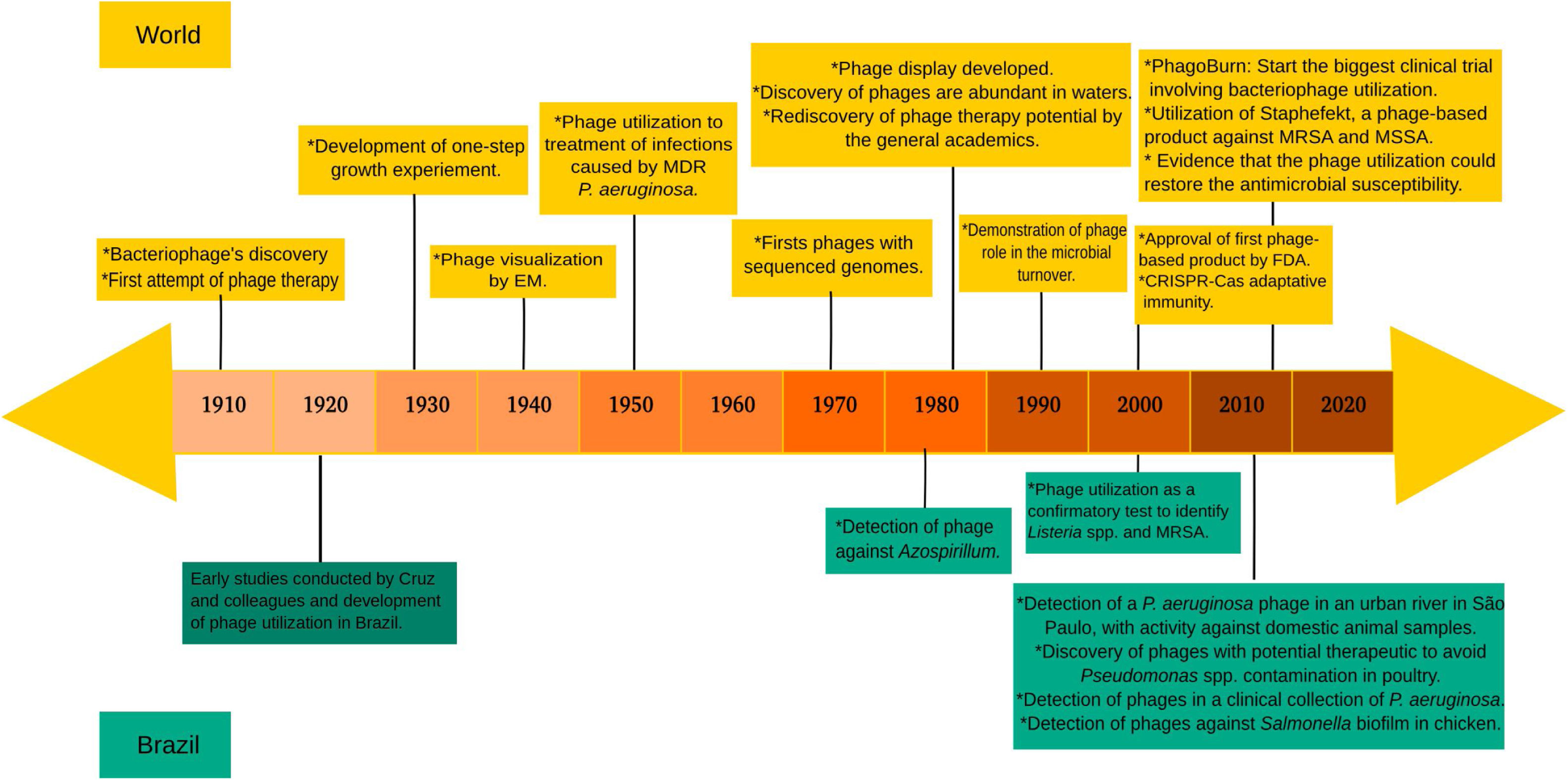
Brazilian studies using phagesUnfortunately, few phage studies have been reported in Brazil. We strongly believe that research in this field is currently under development in Brazil. However, to the best of our knowledge, no clinical studies have been published. Interestingly, a recently published historical review pointed Brazil as one the pioneers of clinical phage utilization against S. aureus and diarrheal bacilli even in the 1920 decade.47 However, after the introduction of antimicrobial agents, and mainly due to discontinuation of research in this field, investigations with bacteriophages were practically abandoned in Brazil.47 Old reports reiterate the Brazilian capacity to invest in bacteriophage technology, aiming at combating bacterial infections. The resumption of studies related to phages in Brazil was started in the last decade and these investigations are related to different fields such as environmental research, veterinary application, human health, and others, though legging behind in terms of type of phages study conducted worldwide (Fig. 2).
Timeline of the main events related to phage's research during the last decades. At the top of the figure, world events related to phage research, while in the bottom, the main researches that were conducted in Brazil. The investigations involving phages in Brazil were obtained through research in the PubMed, using the indicators “Bacteriophages AND Brazil” or “Phages AND Brazil”. For a better understanding or to request more information about events shown on the top of the figure, we suggest reading Salmond and Fineran.14 Additionally, for more information about the early studies carried out in Brazil, we suggest reading Almeida and Sundberg.47
Although more related with contamination of aquatic environment, a well-conducted study about somatic coliphages was carried out in the coastal regions of São Paulo. In this study, it was possible to identify and characterize different types of phages against E. coli isolates. Interestingly, the presence of somatic coliphages is a well-established indication of fecal contamination of the aquatic environment.48
Aiming to perform the decontamination of chicken skin by Salmonella enteritidis, a panel of phages was used with success by researchers of Embrapa in Santa Catarina, Brazil.49 Similar results were observed almost 10 years later by Hungaro et al.,50 who showed the efficacy of phage utilization in reducing Salmonella spp. isolates from chicken skin. Recently, use of phages against P. aeruginosa in the poultry industry was also evidenced in Minas Gerais State.51
A study involving clinical isolates, conducted in São Paulo, detected the presence of phages against clinical isolates of P. aeruginosa. Surprisingly, the phages were isolated from a collection of P. aeruginosa isolates recovered from clinical samples of different hospitals. Some phages showed activity against P. aeruginosa MDR and mucoid isolates. Although a clinical relation had been observed, no data about the clinical utilization of these phages have been reported.52
Recently, a phage with activity against P. aeruginosa was detected in the sewage from Tietê river in São Paulo city. This phage, named BrSP1, was microbiologically and genomically characterized and had its activity tested against P. aeruginosa isolates from domestic animals. BrSP1 phage belonged to the Caudovirales order, a genome with 66,189bp, and had activity against 51.4% of tested isolates. This fact highlights the possibility of phage utilization in human and veterinary medicine.53
Limitation of bacteriophages use in human health and the environmentLike all treatment options, phage utilization also presents limitations that should be considered before clinical use. The absence of large randomized clinical trials, safety evaluation of phage therapy, and the numerous steps necessary before clinical utilization of an identified phage are often reported as the main limitations of this type of therapy. Furthermore, little is known about the role of these bacteriophages in the environment and their capacity to carry resistance genes. To a better understanding of the limitations of phage treatment, we recommend reading Principi et al.54
ConclusionsWe have provided evidence that use of phage therapy could be a new way to combat MDR-pathogens and that this approach would be useful in Brazil where the rates of antibiotic resistance are on the rise. Currently, Brazil is going through an economic crisis, a moment that always requires inexpensive alternatives to tackle different national challenges. In this case, lytic phage utilization could be one of these alternatives, since these agents are simple, inexpensive and abundant in different environments. In addition, more studies need to be supported in this field, aiming to reduce all uncertainties concerning this type of therapy, to gain a new tool in the fight against antimicrobial resistance in Brazil.
FundingW.M.B.S.M. was partially supported by Coordination for the Improvement of the Higher Education Personnel (CAPES) [Process number 88887.32142/2019-00] and fully supported by São Paulo Research Foundation (FAPESP) [Process number 2018/24431-4].
Conflicts of interestA.C.G. has recently received research funding and/or consultation fees from Bayer, Eurofarma, MSD, Pfizer, and Zambon. Other authors have nothing to declare.
We would like to thanks the Coordination for the Improvement of the Higher Education Personnel (CAPES) and the São Paulo Research Foundation (FAPESP) for providing fundings to this researcher.