This retrospective study was conducted to investigate the clinical significance of different Mycoplasma pneumoniae bacterial load in patients with M. pneumoniae pneumonia (MP) in children.
MethodsPatients with MP (n=511) were identified at the Children's Hospital Affiliated to Soochow University database during an outbreak of MP between January 2012 and February 2013.
ResultsComparing patients with high and low bacterial load those with higher loads were significantly older (p<0.01) and had fever significantly more frequently (p=0.01). Presence of wheezing at presentation was associated with low bacterial load (p=0.03). Baseline positive IgM was present in 93 (56.4%) patients with high bacterial load compared to 46 (27.8%) patients with low bacterial load (p<0.001). Co-infection with viruses was found significantly more frequent among patients with low bacterial load (24.2%) than those with high bacterial load (8.5%) [p<0.001]. Bacterial co-infection was also more frequently detected among patients with low bacterial load (22.4%) than in those with high bacterial load (12.1%) [p=0.01].
ConclusionM. pneumoniae at a high bacterial load could be an etiologic agent of respiratory tract disease, whereas the etiologic role of MP at a low bacterial load remains to be determined.
Mycoplasma pneumoniae is an important pathogen responsible for community-acquired pneumonia in children. M. pneumoniae pneumonia (MP) has been reported in 10–40% of community-acquired pneumonia cases.1–4 The mainstay for diagnosing of M. pneumoniae infection presently relies on a rise in paired titer by serological methods.5 Polymerase chain reaction (PCR) has been applied for the early detection of M. pneumoniae infection.6,7 The role of PCR assays performed on upper respiratory tract samples for the diagnosis of M. pneumoniae infection is controversial.5,8 Previous study demonstrated that PCR can detect persistent M. pneumoniae infection up to 7 months after disease onset, the bacterial load in consecutive samples gradually declined in relation to the time interval from onset of illness to sampling.9 Thus, the clinical significance of different M. pneumoniae bacterial load is of much interest.
This study was conducted to investigate the clinical significance of different M. pneumoniae bacterial load using nasopharyngeal aspirates (NPAs) during a recent outbreak of MP in children.
Materials and methodsPatients and specimensThis study was a retrospective analysis of real-time PCR for diagnostic testing of M. pneumoniae infection by antigen detection. Patients hospitalized with clinically and radiologically confirmed lower respiratory tract infections (n=1429) were identified at the Children's Hospital Affiliated to Soochow University database during an outbreak of MP between January 2012 and February 2013. Both serum and NPAs were obtained from these patients. Therefore, patients without stored serum samples or results of NPAs were excluded from our study. Paired serum samples taken on admission and at least seven days after the first collection were available for 733 patients (51.3%).
Real-time PCRNasopharyngeal swabs were obtained within 24h of admission. The specimens were centrifuged and were stored at −80°C until tested. A quantitative diagnostic kit (DaAn Gene Co., Ltd., Guangzhou, China) for M. pneumoniae DNA was used to determine the load of MP, as previously reported.10 The method is based on the TaqMan PCR technology, and the target is 16S rRNA gene specific for MP genome. Briefly, 1mL of nasopharyngeal aspirates diluted with 4% NaOH was centrifuged at 12,000rpm for five min. The sediment was collected, washed twice with 0.9% NaCl, blended with 50μL of DNA extraction solution, incubated at 100°C for 10min, and centrifuged at 12,000rpm for five min. Real-time PCR was performed on the resulting supernatant with 2μL, and PCR mix with 43μL (supplied with the kits) using the DA 7600 real-time PCR system (Applied Biosystems, California, USA) as follows: 93°C for 2min, 10 cycles of 93°C for 45s and 55°C for 60s, followed by 30 cycles of 93°C for 30s and 55°C for 45s. All nasopharyngeal swabs were tested for antigen detection by immunofluorescence for seven common viruses (RSV, adenovirus, influenza viruses A and B, and parainfluenza viruses 1, 2 and 3).
SerologyDetection of serum M. pneumoniae-specific antibody was performed using enzyme-linked immunosorbent assay (Virion-Serion, Germany). The assay was considered positive if IgM≥1.1 or if there was ≥4-fold rise in IgG titer.8
Diagnosis of MPDiagnosis of MP was based on serology or PCR findings. A significant rise in M. pneumoniae IgG titer or the presence of IgM antibodies was used as criteria for current M. pneumoniae infection. Likewise, DNA detection by real-time PCR was also considered M. pneumoniae infection.
Review of medical recordsData compiled from a retrospective chart review of all study patients were extracted through the use of specially prepared data forms without knowledge of serologic status or PCR results. The information extracted included demographics, clinical features and laboratory data.
Statistical analysisWe used n (%) for categorical variables and median (IQR) for continuous variables with non-normal distribution or mean (SD) for those with normal distribution. We assessed differences in categorical variables with the χ2 test. We calculated 95% CI for differences in medians with an exact test. Logistic regression analysis was performed to identify clinical characteristics associated with different bacterial loads. SPSS (version 17.0) software was used for all statistical analysis.
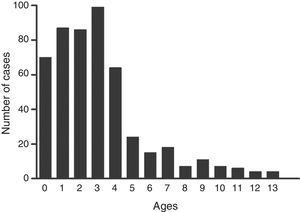
ResultsDemographic findingsAmong 1429 patients with lower respiratory tract infections, 511 (35.8%) were diagnosed with MP based on serology or PCR findings. Of the 511 patients with MP, 304 (59.5%), the mean age was 35 months (range, one month to 156 months). The age distribution of the patients is shown in Fig. 1. The remaining 918 patients who were not diagnosed with MP did not differ significantly from patients with MP in terms of sex and age at disease onset.
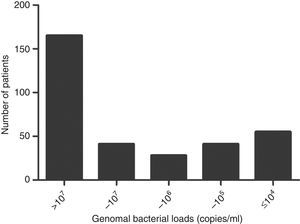
Quantitative analysis of M. pneumoniae DNA in nasopharyngeal aspiratesOf the 511 patients with MP, PCR for M. pneumoniae was positive in 330 (64.6%) nasopharyngeal samples. Genomal bacterial load in NPAs ranged from <2500 to >2.5×107copies/mL of sampled material. According to the distribution of genomal bacterial loads (Fig. 2), patients were classified into two groups: high bacterial load group (>107copies/mL) (n=165) and low bacterial load (<107copies/mL) (n=165).
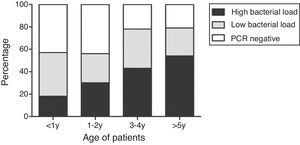
The genomal bacterial load increased significantly with age (p<0.001). Fewer Children younger than one year of age had less high bacterial load (17.7%) than those older than five years (53.8%) [p<0.001] (Fig. 3).
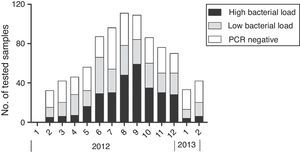
Epidemiology of MP outbreakSeasonal distribution of MP according to genomal bacterial load is shown in Fig. 4.
Outbreaks began in summer months and peaked during August and September (73.5% of all MP cases). The frequency of patients with high bacterial load also increased during summer and peaked in August and September. From January to March 18 (15%) MP patients had high bacterial load, compared to 52 (27.5%) from April to June, 137 (43.3%) from July to September, and 96 (38.7%) from October to December.
Comparison of clinical characteristics by PCR statusClinical characteristics according to PCR status are summarized in Table 1. To assess the independent association of evaluated parameters, mean age, fever, and wheezing at presentation were tested by logistic regression analysis. Patients with high bacterial load were significantly older (p<0.01) and had significantly higher fever (p=0.01). Wheezing at presentation was associated with low bacterial load (p=0.03).
Clinical characteristics according to PCR status in patients with MP.
| Characteristics | Genomal bacterial load | ||
|---|---|---|---|
| PCR negative (n=181) | Low load (n=165) | High load (n=165) | |
| Male:female (n) | 109:72 | 100:65 | 95:70 |
| Mean age (months)a | 26 (29) | 29 (31) | 49 (54)b,d |
| Duration of prior symptoms (days)a | 7.6 (2.5) | 7.5 (5.0) | 7.2 (3.7) |
| Antibiotic therapy within 2 weeks preceding admission, n (%) | 141 (77.9) | 81 (84.4) | 135 (81.8) |
| Fever (Tmax≥38.5°C), n (%) | 111 (61.3) | 97 (58.7) | 127 (77.0)c,d |
| Wheezing at presentation, n (%) | 56 (30.9) | 59 (35.8) | 26 (15.8)c,d |
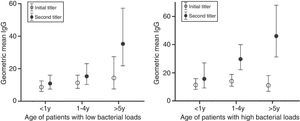
Out of 330 PCR positive children, 179 patients had paired serum samples. No difference was found in the interval between onset of symptoms and the time of obtaining the first serum sample or second serum sample. 93 (56.4%) patients with high bacterial load 46 (27.8%) had more initial positive IgM compared to patients with low bacterial load (p<0.001). Geometric mean titers of initial IgG and IgM of patients with high bacterial load were not significantly different from those of patients with low load. In patients with low bacterial load, geometric mean titers of second IgG significantly increased compared with the initial IgG in patients more than five years old. In patients with high bacterial load, geometric mean titers of second IgG significantly increased in younger patients (Fig. 5). Patients with high bacterial load also had geometric mean titers of second IgM significantly increased in younger patients compared with patients with low bacterial load.
Correlation between initial and second antibody titers among patients with different bacterial loads. Filled circles indicate geometric mean titers (GMTs) of initial antibody titers and unfilled circles indicate GMTs of second antibody titers in each age group and bars indicate 95% confidence intervals.
Co-infections were detected in 204 patients with MP (102 with virus and 102 with bacteria). Co-infection with viruses was significantly more frequent among patients with low bacterial load than those with high bacterial load (24.2% vs. 8.5%; p<0.001). Bacterial co-infections were also more frequently detected among patients with low bacterial load than in those with high bacterial load (22.4% vs. 12.1%; p=0.01).
DiscussionThis study investigated the diagnostic values and clinical significance of real-time PCR in children during an epidemic of M. pneumoniae infection in 2012. M. pneumoniae at a high load was more frequently detected in older children and more strongly associated with fever, but inversely associated with wheezing. In addition, positive initial IgM and significantly increased second antibody titers were detected more often in patients with high bacterial load, which indicated that high bacterial load was associated with increased humoral response.
Various tests have been used to detect MP infection. The mainstay for diagnosing of M. pneumoniae infection presently relies on a rise in Ig titers in paired serum samples.5 However, during the early phase of MP infection, particularly in young children and immunocompromised patients, serological methods have low sensitivity.10,11 In contrast, PCR seemed to be a sensitive, high-throughput and rapid method.12,13 Interestingly, the data presented here demonstrated that the number of children with high bacterial load increased with the age. Only 17.7% of the children with MP younger than one year had high genomal bacterial load, but 53.8% of the children older than five years had high load. Our study also found that positive initial IgM were detected more often in patients with high bacterial load (56.4% vs. 27.8%). In addition, patients with low bacterial load did neither have IgM or IgG increase in the less than one-year and one to four-year-old group when serum paired samples were available.
From culture based studies, it is known that the first step in the pathogenic process of M. pneumoniae involves cytoadherence between M. pneumoniae and respiratory epithelium. After adherence, M. pneumoniae needs to replicate in order to establish an infection, involving colonization and inflammation in human tissues.14 The above findings suggest that in young children M. pneumoniae colonization is usually weak which leads to poor antigenic stimulation. These could explain why antibody response to M. pneumoniae is weak or deferred in young children because of poor antigenic stimulation from another prospective.
Nilsson et al.9 found that the bacterial load in consecutive samples gradually declined in relation to the time interval from onset of illness to sampling. Infections associated with low bacterial load may reflect M. pneumoniae persistence. In our study, 42.1% of the PCR positive children were seropositive. Most of these seropositive diagnoses (93 out of 139, 66.9%) occurred in children with high bacterial load. High bacterial load was noted mainly in the absence of other respiratory viral or bacterial infections, suggesting a causative role of MP. However, low bacterial load was detected more often in viral co-infection and more strongly associated with wheezing, suggesting that a low bacterial load in the nasopharynx seems to be of little clinical significance. Besides, as M. pneumoniae replicates slowly and has limited capacity for protein biosynthesis, the above finding may also suggest that the presence of respiratory viruses may reduce M. pneumoniae colonization. However, we were unable to confirm whether this happened in older children due to the low proportion of mixed infections in age group (3.4% in patients greater than three years).
PCR tests also have their drawbacks. They could not distinguish between viable and nonviable organisms after antibacterial therapy.15–17 Also, concerns exist about the potential inability to distinguish between respiratory carriage vs. active disease.17 Prescribing macrolides in the presence of a positive PCR assay may increase the unnecessary use of macrolides. According to our study, prescribing macrolides based on a positive PCR in patients with low bacterial load and serologically negative should be carefully considered.
The findings of the present study have some limitations. The patients included were hospitalized cases of pneumonia. We did not enroll patients with other spectrum of acute respiratory illnesses. Therefore, patients with more severe symptoms may have been overrepresented. The patients were also more likely to have received antibiotic treatment due to the clinical manifestations.
In conclusion, our results suggest that M. pneumoniae at a high bacterial load could be an etiologic agent of respiratory tract disease, whereas the etiologic role of MP at a low bacterial load remains to be determined. Quantitative analysis may, therefore, be important for studies of M. pneumoniae infection.
Conflicts of interestThe authors declare no conflicts of interest.