Bacteremic pneumococcal pneumonia (BPP) is a severe condition. To evaluate seasonal distribution, mortality, serotype frequencies, antimicrobial susceptibility, and different severity scores among patients with BPP.
Patients and methodsPatients were identified by laboratory data and restricted to adulthood. Standard methods were used for serotyping and antimicrobial susceptibility. Risk factors were analyzed by univariate and multivariate methods. Severity scores (APACHE II, CURB-65 and CAP PIRO) were compared using ROC curves.
ResultsSixty events of community-acquired BPP occurred between 2005 and 2010. A seasonal pattern was detected. Mean age was 72.1 years old (81.4% ≥60 years). All had a predisposing factor. Previous influenza (3.3%) or pneumococcal immunization (1.7%) was infrequent. Admission to critical units was required by 51.7%. Twenty-two serotypes were identified among 59 strains. Only one strain had intermediate resistance to penicillin (1.7%). In-hospital mortality reached 33.3%. Multivariate analysis identified a CAP PIRO score>3 (OR 29.7; IC95 4.7–187), age ≥65 years (OR 42.1; IC95 2.2–796), and a platelet count<100,000/μL (OR 10.9; IC95 1.2–96) as significant independent factors associated with death. ROC curve analysis did not reveal statistical differences between the three severity scores to predict death (AUC 0.77–0.90). The prognostic yield for all of them was limited (Positive Likelihood Ratio: 1.5–3.8).
ConclusionsBPP had a high case-fatality rate in this group of adult patients with no association to resistant isolates, and a low immunization record. Three independent factors were related to death and the prognostic yield of different severity scores was low.
Invasive infections due to Streptococcus pneumoniae are an important cause or morbidity and mortality with a high disease burden.1 In addition, invasive disease cases will probably increase in the near future due to population aging.2 Bacteremic pneumococcal pneumonia represents an invasive disease that is linked to extreme ages and comorbidities.3,4 It has sometimes been associated with a range of different serotypes, antibiotic resistance and a high case-fatality ratio.3,5–7 Updated information about antibiotic resistance and serotype distribution is needed for antimicrobial treatment and to evaluate vaccine efficacy. However, data regarding serotype frequencies is scarce in adults in several countries of Latin America because it has mainly been focused on children.8,9 In addition, different severity scores have been applied in patient with community-acquired pneumonia or in those seriously-ill4,10,11 but not in a specific group of patients such as those with bacteremic pneumococcal pneumonia. We report the result of a 6-year period retrospective study in patients with bacteremic pneumococcal pneumonia. The aims were to examine seasonal distribution, patient’ characteristics, mortality rate, use of intensive care resources, serotype frequencies, antimicrobial susceptibility, and severity scores prediction yield. Risk factors associated to critical care unit admission and mortality, were also explored.
Patients and methodsDesign of study and settingRetrospective and descriptive study performed at the Hospital Militar de Santiago, Chile. This institution is a 250-bed general hospital for active or retired military personnel and their relatives.
Inclusion criteria and variables includedEvery adult patient≥18 years, admitted between 2005 and 2010 with S. pneumoniae growing in blood cultures, and with respiratory symptoms and/or pneumonia was included. Potential cases were identified using the microbiology laboratory database. Medical charts were reviewed and data on the following variables were obtained: co-morbidities (pulmonary diseases, cardiac disease, renal failure, diabetes mellitus), immunosuppression, alcoholism or tobacco consumption, influenza and pneumococcal vaccination coverage, clinical manifestations, physical examination, laboratory values, chest X-ray and/or CT information, admission to intermediate or critical care unit, use of vasoactive drugs or shock, antimicrobial therapy, steroid use during the first 72h of admission, length of stay (LOS) and hospital mortality. APACHE II, CURB-65 and CAP-PIRO severity scores were applied.10,11 Data on antimicrobial susceptibility and serotyping were included. The latter was performed in the national reference laboratory. The following operative definitions were applied: tobacco consumption (any quantity of cigarettes per day in the last two years), alcohol consumption (any quantity of ethanol consumption per day), shock (arterial systolic pressure<90mmHg with no response to fluid resuscitation or need for vasoactive drugs), acute renal injury (serum creatinine level>1.5mg/dL at admission or 50% increase above the baseline value), acute respiratory distress syndrome (ARDS: PaFiO2<200 and bilateral infiltrates), and complicated pleural effusion (purulent pleural fluid or bacteria on gram stain, requiring a chest tube or surgical drainage).
Statistical analysisClinical information is presented in a descriptive manner. Continuous variables were categorized to facilitate analyses. Potential factors linked to in-hospital mortality or intermediate-intensive care unit admission, were explored using chi-square or two-sided Fisher's exact test. Univariate analysis including odds ratios (OR) was performed, adding 0.5 to every cell in the 2×2 contingency table when null cells were present. Multivariate analysis was developed through binary logistic regression. ROC curves were applied in order to compare different numerical variables associated to higher in-hospital mortality, and cutoff values were established. The areas under the curve (AUC) from different predictive scales were compared using Epidat software.
ResultsGeneral featuresFrom 2005 to 2010, sixty events of community-acquired bacteremic pneumococcal pneumonia occurred in 59 patients. One patient had two events three years apart from each other. In this case demographics, tobacco smoking, alcohol consumption and co-morbidities were counted once, but data on clinical manifestations, laboratory, radiologic, and vaccine coverage, were counted separately.
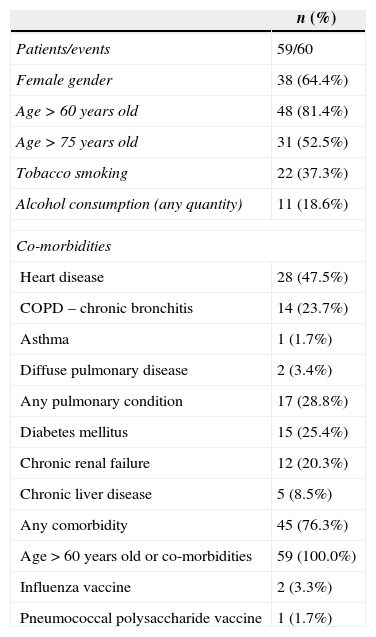
Near two-thirds of patients were females. Mean age was 72.1 years old (range 20–96; Table 1). Over 80% of the subjects was 60 years or older, and half was older than 75 years. In addition, one third declared tobacco smoking and almost 20% were alcohol consumers but a detailed characterization on the quantity used was not possible. Medical morbid conditions were frequent in this series, heart disease, different pulmonary conditions, diabetes mellitus, and chronic renal failure predominated. All patients had a predisposing condition and 76% had co-morbidities. No patients with HIV infection or solid or bone marrow transplantation were identified in this series, and influenza or pneumococcal vaccine coverage was exceedingly rare (Table 1).
General features among patients with community-acquired bacteremic pneumococcal pneumonia admitted to Hospital Militar de Santiago, 2005–2010.
| n (%) | |
|---|---|
| Patients/events | 59/60 |
| Female gender | 38 (64.4%) |
| Age>60 years old | 48 (81.4%) |
| Age>75 years old | 31 (52.5%) |
| Tobacco smoking | 22 (37.3%) |
| Alcohol consumption (any quantity) | 11 (18.6%) |
| Co-morbidities | |
| Heart disease | 28 (47.5%) |
| COPD – chronic bronchitis | 14 (23.7%) |
| Asthma | 1 (1.7%) |
| Diffuse pulmonary disease | 2 (3.4%) |
| Any pulmonary condition | 17 (28.8%) |
| Diabetes mellitus | 15 (25.4%) |
| Chronic renal failure | 12 (20.3%) |
| Chronic liver disease | 5 (8.5%) |
| Any comorbidity | 45 (76.3%) |
| Age>60 years old or co-morbidities | 59 (100.0%) |
| Influenza vaccine | 2 (3.3%) |
| Pneumococcal polysaccharide vaccine | 1 (1.7%) |
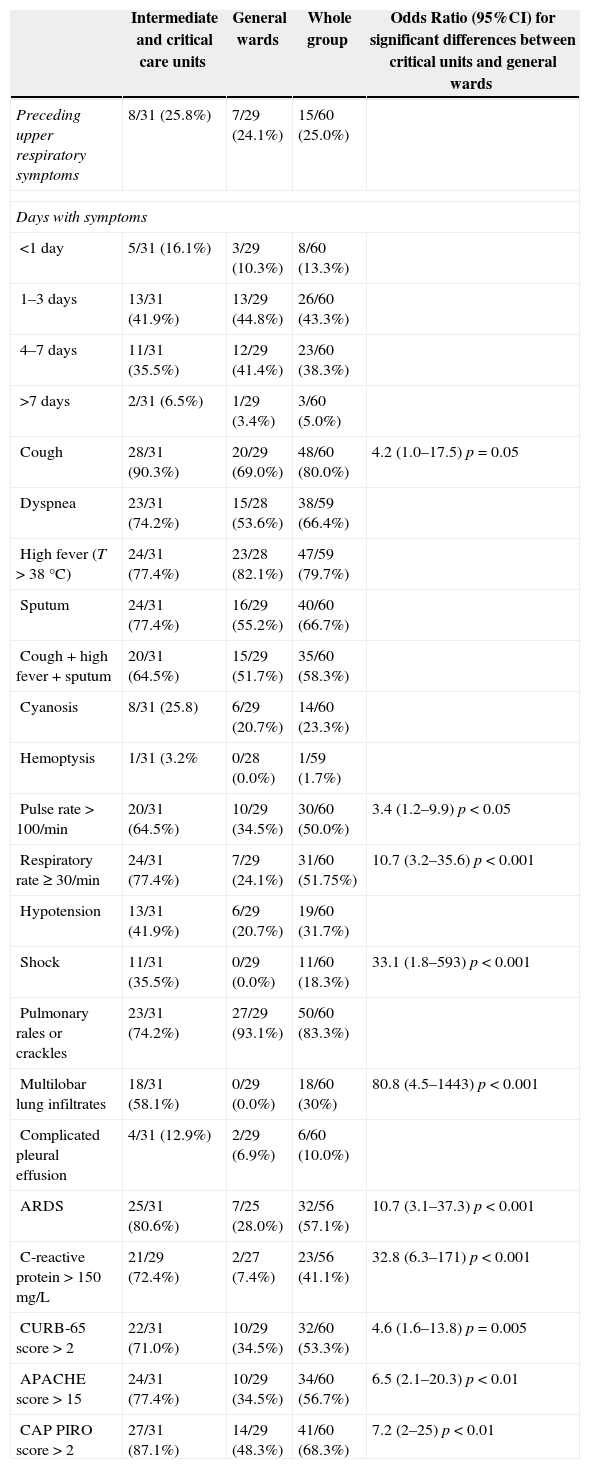
Bacteremic events were characterized by a severe clinical condition reflected in the relative higher frequency of cyanosis, respiratory rate>30min–1, and hypotension (Table 2, whole group column). More than half had an APACHE II score>15 points and two-thirds a CAP PIRO score>2. Cough, fever, and lung sounds in the form of rales or crackles were frequent (∼80%, Table 2, whole group). Most patients presented one to seven days of symptoms before admission, but near 15% of events started on the same day of hospitalization (Table 2, whole group).
Clinical manifestations and severity scores at admission of 60 events of bacteremic pneumococcal pneumonia. Hospital Militar de Santiago, 2005–2010.
| Intermediate and critical care units | General wards | Whole group | Odds Ratio (95%CI) for significant differences between critical units and general wards | |
|---|---|---|---|---|
| Preceding upper respiratory symptoms | 8/31 (25.8%) | 7/29 (24.1%) | 15/60 (25.0%) | |
| Days with symptoms | ||||
| <1 day | 5/31 (16.1%) | 3/29 (10.3%) | 8/60 (13.3%) | |
| 1–3 days | 13/31 (41.9%) | 13/29 (44.8%) | 26/60 (43.3%) | |
| 4–7 days | 11/31 (35.5%) | 12/29 (41.4%) | 23/60 (38.3%) | |
| >7 days | 2/31 (6.5%) | 1/29 (3.4%) | 3/60 (5.0%) | |
| Cough | 28/31 (90.3%) | 20/29 (69.0%) | 48/60 (80.0%) | 4.2 (1.0–17.5) p=0.05 |
| Dyspnea | 23/31 (74.2%) | 15/28 (53.6%) | 38/59 (66.4%) | |
| High fever (T>38°C) | 24/31 (77.4%) | 23/28 (82.1%) | 47/59 (79.7%) | |
| Sputum | 24/31 (77.4%) | 16/29 (55.2%) | 40/60 (66.7%) | |
| Cough+high fever+sputum | 20/31 (64.5%) | 15/29 (51.7%) | 35/60 (58.3%) | |
| Cyanosis | 8/31 (25.8) | 6/29 (20.7%) | 14/60 (23.3%) | |
| Hemoptysis | 1/31 (3.2% | 0/28 (0.0%) | 1/59 (1.7%) | |
| Pulse rate>100/min | 20/31 (64.5%) | 10/29 (34.5%) | 30/60 (50.0%) | 3.4 (1.2–9.9) p<0.05 |
| Respiratory rate≥30/min | 24/31 (77.4%) | 7/29 (24.1%) | 31/60 (51.75%) | 10.7 (3.2–35.6) p<0.001 |
| Hypotension | 13/31 (41.9%) | 6/29 (20.7%) | 19/60 (31.7%) | |
| Shock | 11/31 (35.5%) | 0/29 (0.0%) | 11/60 (18.3%) | 33.1 (1.8–593) p<0.001 |
| Pulmonary rales or crackles | 23/31 (74.2%) | 27/29 (93.1%) | 50/60 (83.3%) | |
| Multilobar lung infiltrates | 18/31 (58.1%) | 0/29 (0.0%) | 18/60 (30%) | 80.8 (4.5–1443) p<0.001 |
| Complicated pleural effusion | 4/31 (12.9%) | 2/29 (6.9%) | 6/60 (10.0%) | |
| ARDS | 25/31 (80.6%) | 7/25 (28.0%) | 32/56 (57.1%) | 10.7 (3.1–37.3) p<0.001 |
| C-reactive protein>150mg/L | 21/29 (72.4%) | 2/27 (7.4%) | 23/56 (41.1%) | 32.8 (6.3–171) p<0.001 |
| CURB-65 score>2 | 22/31 (71.0%) | 10/29 (34.5%) | 32/60 (53.3%) | 4.6 (1.6–13.8) p=0.005 |
| APACHE score>15 | 24/31 (77.4%) | 10/29 (34.5%) | 34/60 (56.7%) | 6.5 (2.1–20.3) p<0.01 |
| CAP PIRO score>2 | 27/31 (87.1%) | 14/29 (48.3%) | 41/60 (68.3%) | 7.2 (2–25) p<0.01 |
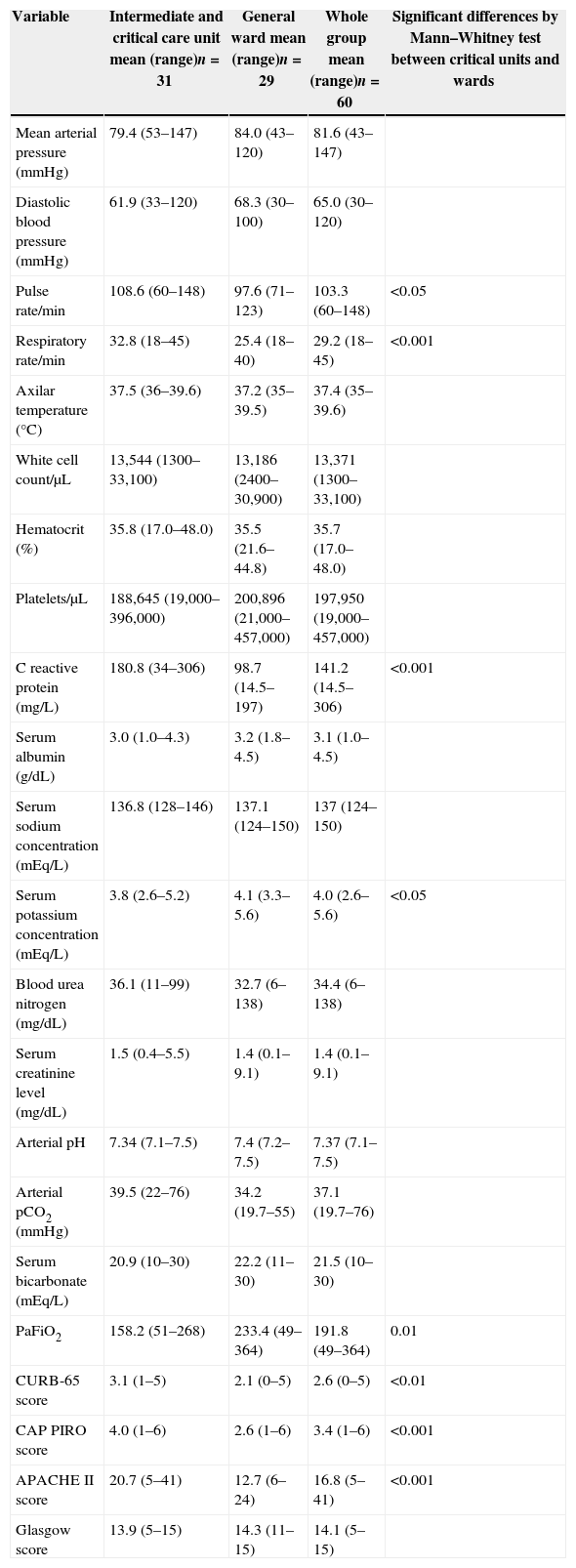
As a group (Table 3, whole group column), patients with bacteremic pneumococcal pneumonia presented tachycardia, tachypnea, fever, leucocytosis, elevated C reactive protein, low serum albumin concentration, and respiratory failure with low PaFiO2 index together with increased values of blood urea nitrogen and serum creatinine concentration. Ten percent had complicated pleural effusion and required a chest tube or surgical drainage. Thirty percent presented multilobar lung infiltrates and 57% ARDS (Table 2, whole group).
Physical exam parameters, laboratory values and severity scores results among 60 events of bacteremic pneumococcal pneumonia, Hospital Militar de Santiago, 2005–2010.
| Variable | Intermediate and critical care unit mean (range)n=31 | General ward mean (range)n=29 | Whole group mean (range)n=60 | Significant differences by Mann–Whitney test between critical units and wards |
|---|---|---|---|---|
| Mean arterial pressure (mmHg) | 79.4 (53–147) | 84.0 (43–120) | 81.6 (43–147) | |
| Diastolic blood pressure (mmHg) | 61.9 (33–120) | 68.3 (30–100) | 65.0 (30–120) | |
| Pulse rate/min | 108.6 (60–148) | 97.6 (71–123) | 103.3 (60–148) | <0.05 |
| Respiratory rate/min | 32.8 (18–45) | 25.4 (18–40) | 29.2 (18–45) | <0.001 |
| Axilar temperature (°C) | 37.5 (36–39.6) | 37.2 (35–39.5) | 37.4 (35–39.6) | |
| White cell count/μL | 13,544 (1300–33,100) | 13,186 (2400–30,900) | 13,371 (1300–33,100) | |
| Hematocrit (%) | 35.8 (17.0–48.0) | 35.5 (21.6–44.8) | 35.7 (17.0–48.0) | |
| Platelets/μL | 188,645 (19,000–396,000) | 200,896 (21,000–457,000) | 197,950 (19,000–457,000) | |
| C reactive protein (mg/L) | 180.8 (34–306) | 98.7 (14.5–197) | 141.2 (14.5–306) | <0.001 |
| Serum albumin (g/dL) | 3.0 (1.0–4.3) | 3.2 (1.8–4.5) | 3.1 (1.0–4.5) | |
| Serum sodium concentration (mEq/L) | 136.8 (128–146) | 137.1 (124–150) | 137 (124–150) | |
| Serum potassium concentration (mEq/L) | 3.8 (2.6–5.2) | 4.1 (3.3–5.6) | 4.0 (2.6–5.6) | <0.05 |
| Blood urea nitrogen (mg/dL) | 36.1 (11–99) | 32.7 (6–138) | 34.4 (6–138) | |
| Serum creatinine level (mg/dL) | 1.5 (0.4–5.5) | 1.4 (0.1–9.1) | 1.4 (0.1–9.1) | |
| Arterial pH | 7.34 (7.1–7.5) | 7.4 (7.2–7.5) | 7.37 (7.1–7.5) | |
| Arterial pCO2 (mmHg) | 39.5 (22–76) | 34.2 (19.7–55) | 37.1 (19.7–76) | |
| Serum bicarbonate (mEq/L) | 20.9 (10–30) | 22.2 (11–30) | 21.5 (10–30) | |
| PaFiO2 | 158.2 (51–268) | 233.4 (49–364) | 191.8 (49–364) | 0.01 |
| CURB-65 score | 3.1 (1–5) | 2.1 (0–5) | 2.6 (0–5) | <0.01 |
| CAP PIRO score | 4.0 (1–6) | 2.6 (1–6) | 3.4 (1–6) | <0.001 |
| APACHE II score | 20.7 (5–41) | 12.7 (6–24) | 16.8 (5–41) | <0.001 |
| Glasgow score | 13.9 (5–15) | 14.3 (11–15) | 14.1 (5–15) |
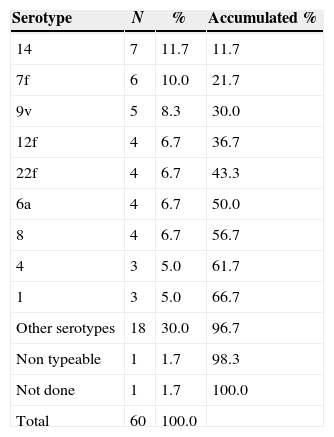
Twenty-two serotypes were identified among 59 strains. One isolate was untypeable and one strain was not analyzed. Two-thirds of the sample were represented by nine predominant serotypes (14, 7f, 9v, 12f, 22f, 6a, 8, 4, and 1) (Table 4). Other 13 serotypes were detected once or twice in this period (23a, 23f, 5, 6b, 13, 15f, 17f, 18f, 19a, 3, 9n, poolr, 33f). Most frequent serotypes were evenly distributed through the whole period. The same serotype was found in both isolates from the patient with two bacteremic episodes (serotype 5).
Serotype distribution among 59 strains of Streptococcus pneumoniae isolated during bacteremic events.
| Serotype | N | % | Accumulated % |
|---|---|---|---|
| 14 | 7 | 11.7 | 11.7 |
| 7f | 6 | 10.0 | 21.7 |
| 9v | 5 | 8.3 | 30.0 |
| 12f | 4 | 6.7 | 36.7 |
| 22f | 4 | 6.7 | 43.3 |
| 6a | 4 | 6.7 | 50.0 |
| 8 | 4 | 6.7 | 56.7 |
| 4 | 3 | 5.0 | 61.7 |
| 1 | 3 | 5.0 | 66.7 |
| Other serotypes | 18 | 30.0 | 96.7 |
| Non typeable | 1 | 1.7 | 98.3 |
| Not done | 1 | 1.7 | 100.0 |
| Total | 60 | 100.0 |
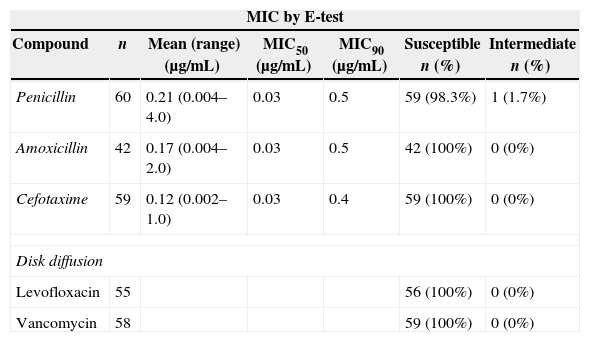
E-test and disk diffusion were used to study antimicrobial susceptibility (Table 5). MIC50 and MIC90 values for penicillin, amoxicillin and cefotaxime were remarkably low and only one strain with intermediate susceptibility to penicillin was detected. No resistant isolates to levofloxacin or vancomycin were identified by the disk diffusion method.
Antimicrobial susceptibility studies.
| MIC by E-test | ||||||
|---|---|---|---|---|---|---|
| Compound | n | Mean (range) (μg/mL) | MIC50 (μg/mL) | MIC90 (μg/mL) | Susceptible n (%) | Intermediate n (%) |
| Penicillin | 60 | 0.21 (0.004–4.0) | 0.03 | 0.5 | 59 (98.3%) | 1 (1.7%) |
| Amoxicillin | 42 | 0.17 (0.004–2.0) | 0.03 | 0.5 | 42 (100%) | 0 (0%) |
| Cefotaxime | 59 | 0.12 (0.002–1.0) | 0.03 | 0.4 | 59 (100%) | 0 (0%) |
| Disk diffusion | ||||||
| Levofloxacin | 55 | 56 (100%) | 0 (0%) | |||
| Vancomycin | 58 | 59 (100%) | 0 (0%) | |||
Thirty-one patients were admitted to an intermediate or critical care unit (51.7%). Thirty per cent of bacteremic events required mechanical ventilation. Antimicrobial therapy was started three to four days after symptoms appeared (range 0–21, median 3, IQR 1–5 days). All events were adequately treated from the start according to susceptibility studies. In addition, 40.7% (n=24) received corticosteroid therapy during the first 72h of hospitalization. The mean accumulated dose for this period was equivalent of 151mg of prednisone (range 38–650mg). In-hospital mortality reached 33.3% (20 out of 60 bacteremic events). Length of stay ranged from one to 93 days (mean 13.3 days).
Risk factors associated to admission to intermediate or critical care unitUnivariate analysis identified several factors significantly associated with admission to intermediate or critical care unit. Categorical variables such as tobacco smoking (OR 3.1; 95% CI 1.0–9.5, p<0.05), cough (OR 4.2; 95% CI 1.0–17.5, p=0.05), tachycardia (OR 3.4; 95% CI 1.2–9.9, p<0.05), respiratory rate>30min–1 (OR 10.7; 95% CI 3.2–35.6, p<0.001), shock (OR 33.1; 95% CI 1.8–593, p<0.001), multilobar infiltrates (OR 80.8; 95% CI 4.5–1443, p<0.001), ARDS (OR 10.7; 95% CI 3.1–37.3, p<0.001), C-reactive protein>150mg/dL (OR 32.8; 95% CI 6.3–171, p<0.001), APACHE II score>15 (OR 6.5; 95% CI 2.1–20.3, p<0.01), CURB-65 score>2 (OR 4.6; 95% CI 1.6–13.8, p=0.005), and CAP PIRO score>2 (OR 7.2; 95% CI 2–25, p<0.01) (Table 2) were associated to hospitalization in critical units. This association was also established for the following continuous variables for higher values: pulse and respiratory rate, C-reactive protein, APACHE II score, CURB-65, CAP PIRO score (Table 3), and for lower values of PaFiO2 index and serum potassium concentration (Table 3). Multivariate analysis indicated that only a C-reactive value >150mg/L was independently associated with admission to these units (OR 17.6; 95% CI 1.8–109, p<0.01).
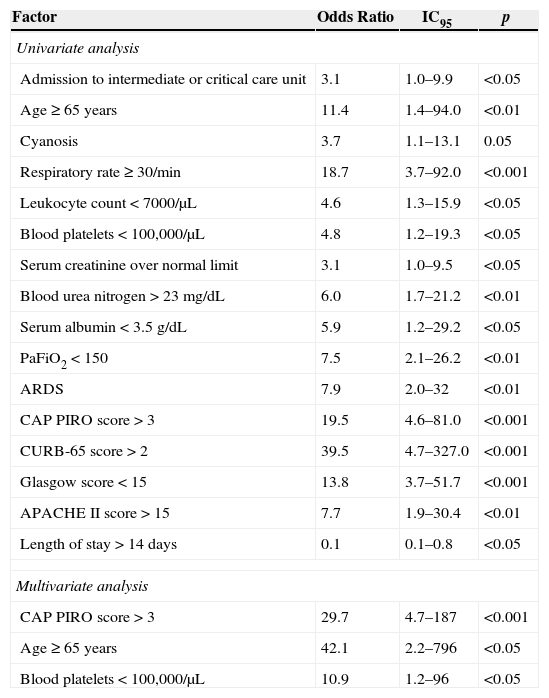
Risk factors associated to in-hospital mortalityTwenty patients died in this series and univariate analysis identified several associations (Table 6). Most relevant factors were CURB-65 score>2 (OR 39.5; 95% CI 4.7–327.0), CAP PIRO score>3 (OR 19.5; 95% CI 4.6–81.0), respiratory rate>30 (OR 18.7; 95% CI 3.7–92.0), Glasgow score<15 (OR 13.8; 95% CI 3.7–51.7), and age>65 years (OR 11.4; 95% CI 1.4–94.0). A LOS>14 days was significantly associated with reduced in-hospital mortality (OR 0.1; 95% CI 0.1–0.8). Corticosteroid therapy was not linked to any particular effect. Multivariate analysis identified CAP PIRO score>3, age≥65 years, and platelet count <100,000/μL as independent factors linked to higher in-hospital mortality (Table 6).
Risk factors associated to in-hospital mortality among 60 events of bacteremic pneumococcal pneumonia. Hospital Militar de Santiago, 2005–2010.
| Factor | Odds Ratio | IC95 | p |
|---|---|---|---|
| Univariate analysis | |||
| Admission to intermediate or critical care unit | 3.1 | 1.0–9.9 | <0.05 |
| Age≥65 years | 11.4 | 1.4–94.0 | <0.01 |
| Cyanosis | 3.7 | 1.1–13.1 | 0.05 |
| Respiratory rate≥30/min | 18.7 | 3.7–92.0 | <0.001 |
| Leukocyte count<7000/μL | 4.6 | 1.3–15.9 | <0.05 |
| Blood platelets<100,000/μL | 4.8 | 1.2–19.3 | <0.05 |
| Serum creatinine over normal limit | 3.1 | 1.0–9.5 | <0.05 |
| Blood urea nitrogen>23mg/dL | 6.0 | 1.7–21.2 | <0.01 |
| Serum albumin<3.5g/dL | 5.9 | 1.2–29.2 | <0.05 |
| PaFiO2<150 | 7.5 | 2.1–26.2 | <0.01 |
| ARDS | 7.9 | 2.0–32 | <0.01 |
| CAP PIRO score>3 | 19.5 | 4.6–81.0 | <0.001 |
| CURB-65 score>2 | 39.5 | 4.7–327.0 | <0.001 |
| Glasgow score<15 | 13.8 | 3.7–51.7 | <0.001 |
| APACHE II score>15 | 7.7 | 1.9–30.4 | <0.01 |
| Length of stay>14 days | 0.1 | 0.1–0.8 | <0.05 |
| Multivariate analysis | |||
| CAP PIRO score>3 | 29.7 | 4.7–187 | <0.001 |
| Age ≥ 65 years | 42.1 | 2.2–796 | <0.05 |
| Blood platelets<100,000/μL | 10.9 | 1.2–96 | <0.05 |
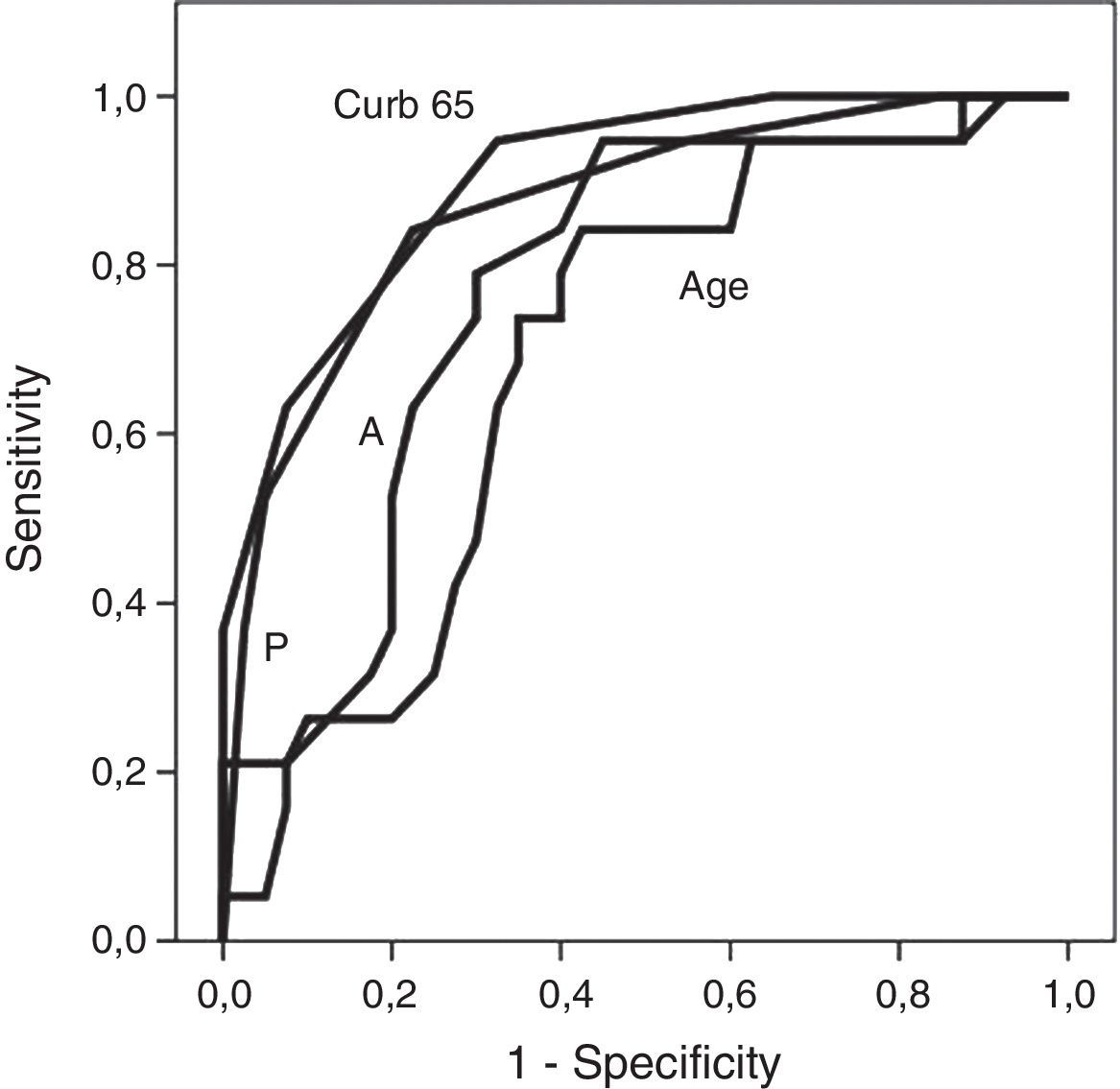
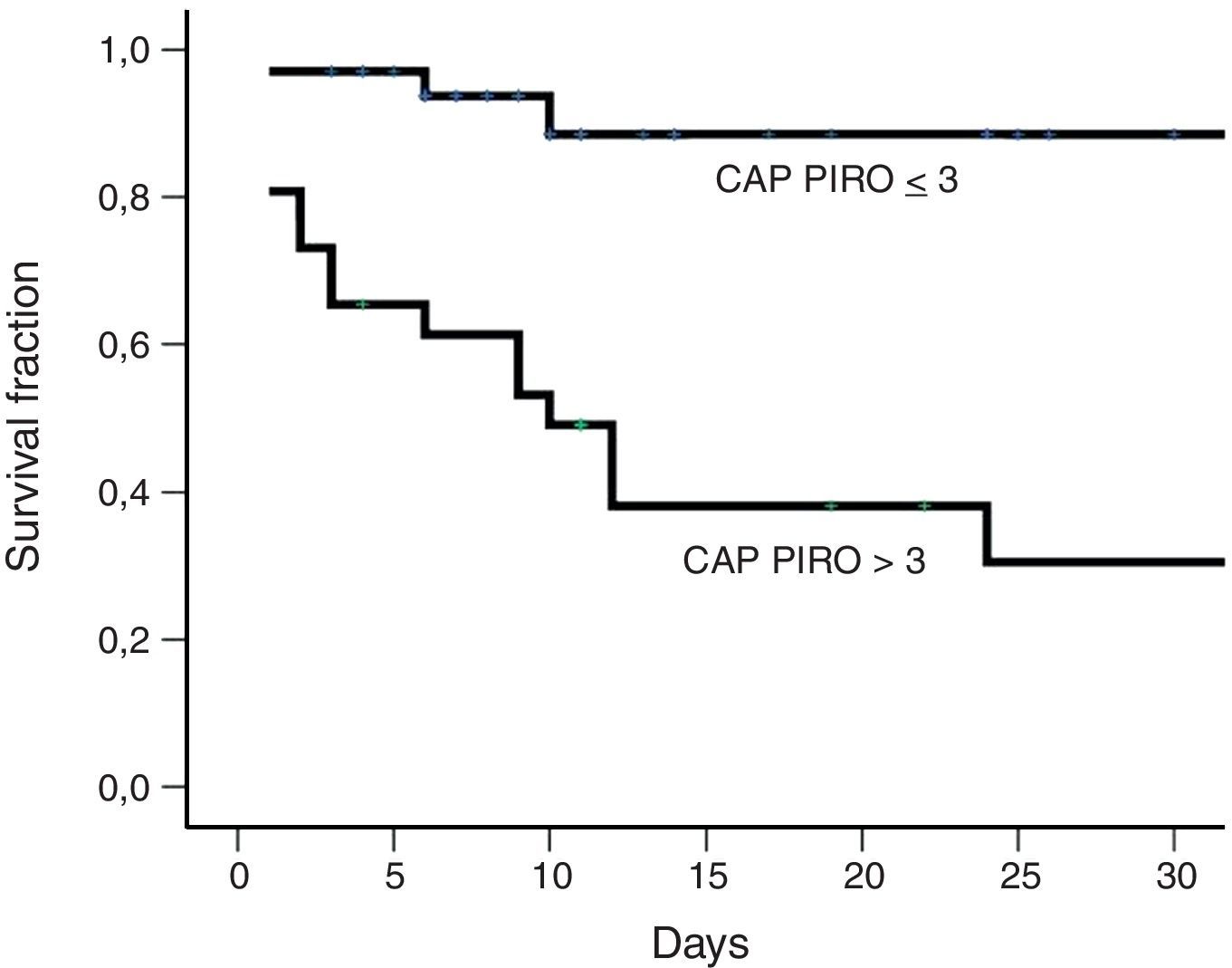
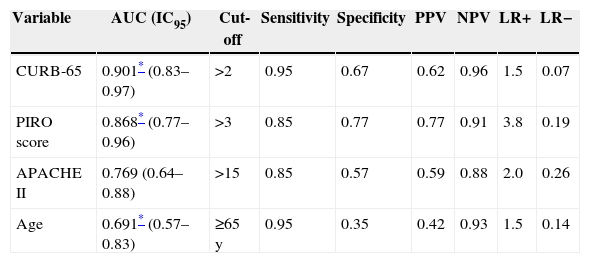
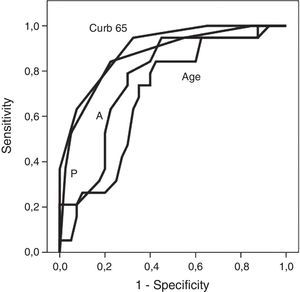
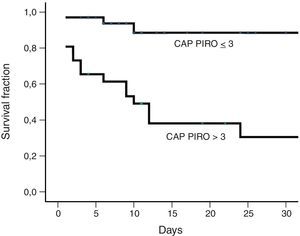
Fig. 1 shows receiver operating characteristic curves for in-hospital mortality according to PIRO, APACHE II, CURB-65 scores and age. CURB-65 and CAP PIRO scores surpassed the area under the curve (AUC) prediction obtained with APACHE II or Age but matched between them (Table 7). Significant statistical differences were found between the AUC of CURB-65 and age (p<0.01); also with CAP PIRO versus age (p<0.05). Survival curves at 30 day since admission using a stratified CAP PIRO score (≤3 or >3) was statistically significant (Log rank test p<0.001) (Fig. 2). Cut-off values optimized with ROC analysis and different statistics parameters are displayed in Table 7. Sensitivity and specificity regarding prognostic yield was limited. Severity scores were more useful as negative predictive values if the patient was below the cut-off.
Cut-off values and diagnostic parameters for different severity scores and age as predictors of in-hospital mortality.
| Variable | AUC (IC95) | Cut-off | Sensitivity | Specificity | PPV | NPV | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|
| CURB-65 | 0.901* (0.83–0.97) | >2 | 0.95 | 0.67 | 0.62 | 0.96 | 1.5 | 0.07 |
| PIRO score | 0.868* (0.77–0.96) | >3 | 0.85 | 0.77 | 0.77 | 0.91 | 3.8 | 0.19 |
| APACHE II | 0.769 (0.64–0.88) | >15 | 0.85 | 0.57 | 0.59 | 0.88 | 2.0 | 0.26 |
| Age | 0.691* (0.57–0.83) | ≥65 y | 0.95 | 0.35 | 0.42 | 0.93 | 1.5 | 0.14 |
PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio.
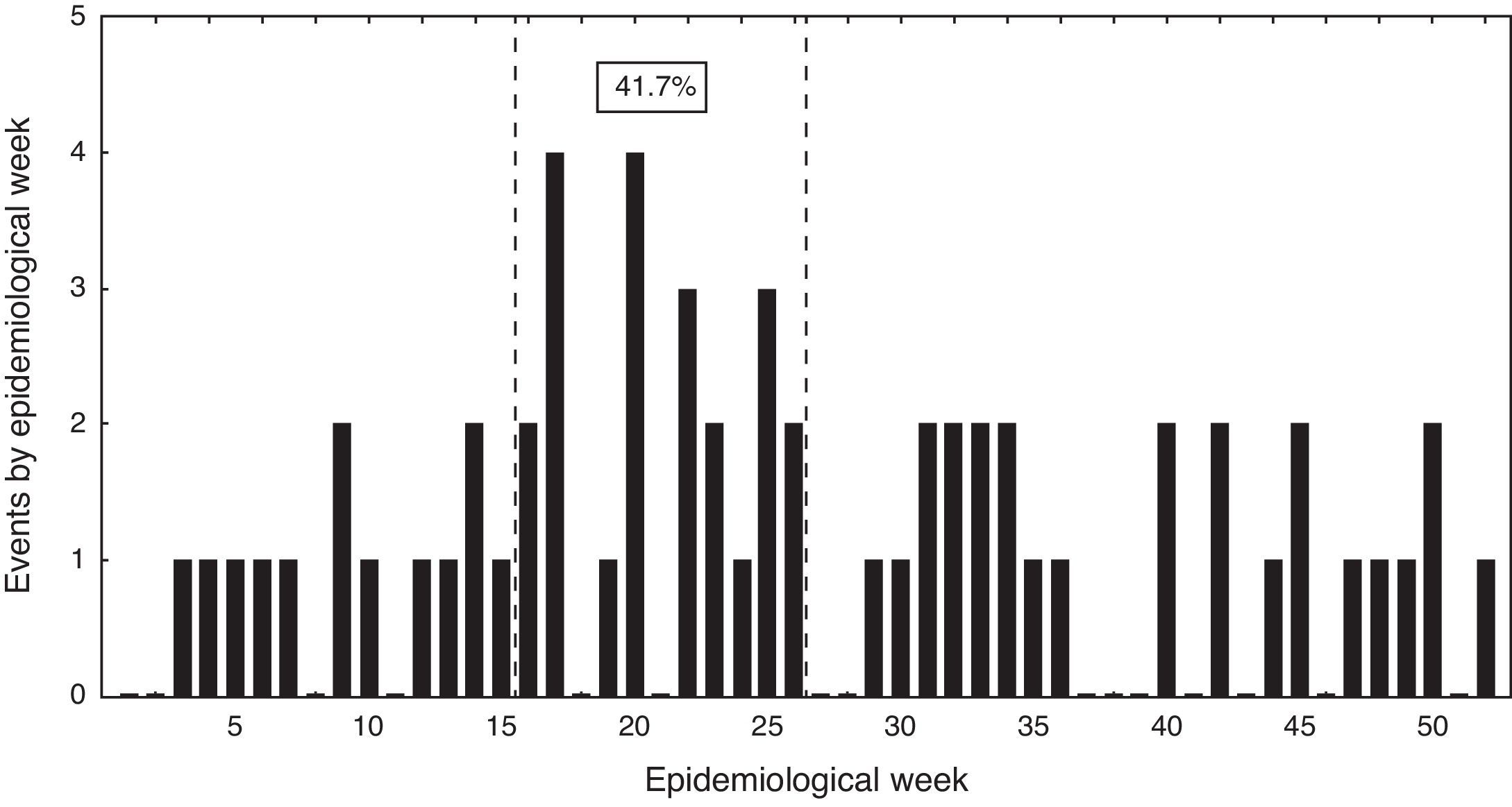
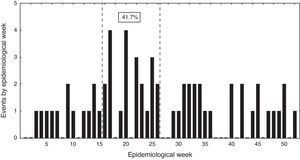
Events distribution showed a sporadic pattern along the study ranging from 4 to 17 cases per year. There was a non-significant trend toward case reduction during this period (Pearson correlation coefficient −0.46; p>0.05). Over 40% of cases were hospitalized between epidemiological weeks 14 and 26 (Fig. 3).
DiscussionThe aims of this study were to evaluate clinical, epidemiological, and microbiological features, as well as predictors of mortality and critical care unit admission in a group of adult patients hospitalized for bacteremic pneumococcal pneumonia. Several findings deserve attention.
From an epidemiological perspective, a seasonal pattern was observed with a higher incidence of cases during mid-fall and early winter (weeks 14–26). This phenomenon had been previously described in adults, associated to a preceding viral respiratory infection and air pollution,12 and peak incidence of bacteremic events coinciding with seasonal influenza in Santiago.13 Between 2000 and 2010 a decreasing trend of these bacteremic events was observed in parallel to a reduction in mortality due to pneumonia among the Chilean elderly patients (Pearson coefficient −0.70, data not shown).14 Death rate due to pneumonia decreased from 324 to 222 per 100,000 inhabitants in this age group, and it is possible that the decline observed in our work is somehow mirroring a global trend in Chile. This change is probably related to multiple factors, like, a steadily increase in influenza vaccine doses. At present, 25% of the Chilean population is being immunized for influenza (over 4 million doses). In addition, since 2009 a national polysaccharide 23-valent pneumococcal immunization program is applied among elderly people, albeit with a reduced coverage of the targeted population (near 6%). Moreover, during the last decade a significant reduction in air pollution has occurred in the metropolitan area,15 and a timely, low cost access to medical attention and antimicrobial therapy is guaranteed for all aged patients with ATS II community acquired pneumonia. These interventions could enhance the decreasing trend of this condition in the future. Infant immunization with pneumococcal conjugated vaccines can influence the frequency of invasive pneumococcal disease in adults through its effect on colonization and transmission.16 A national immunization program for infants with a 10-valent conjugated pneumococcal vaccine was launched in Chile at the end of 2010, but beyond the study period and could have not influenced on this decrease.
Three quarters of our patients had co-morbidities and all had an older age or chronic diseases. The high prevalence of co-morbid conditions is a common feature in adult patients affected by pneumococcal pneumonia with or without bacteremia, underscoring the importance of host factors in disease progression, and at the same time, probably explaining why the bacteremic condition has not been clearly associated with a worst prognosis than cases without this condition.4,5,17,18 The death toll observed in our work (33%) is in line with host characteristic (30%) and similar to figures reported in one group from Brazil.5 However, it is higher than previous reports from Chile (10.9 and 20%).3,4 This could be related to the older age range of our patients.
Most of the patients had a severe underlying clinical condition and almost 15% suffered an abrupt onset. Cyanosis, a respiratory rate>30/min, and hypotension were relatively frequent. Laboratory parameters included renal (BUN ∼30mg/dL) and respiratory failure (PaFiO2 ∼190), leukocytosis (∼13,000/μL), elevated C-reactive protein (∼140mg/L) and low serum albumin (∼3g/dL). In addition, multilobar infiltrates were detected in almost one third of the subjects and 10% required a chest tube or surgery due to pleural effusion. These findings were also common in other series.3–5
Several risk factors have been associated to a higher risk of death in adult patients with pneumococcal infections. However, results vary depending on the type of infection (pneumonia, bacteremic or non-bacteremic conditions, meningitis), group of patients (general, HIV-infected, liver disease, cancer patients), and comparison groups (influenza, patients affected by CAP but without microbiological diagnosis, etc.).3–5,17–21 A common finding is the higher risk associated to older age, co-morbidities and severity of infection. Death rate in the bacteremic subgroup has been related to male gender, renal failure, acidosis, shock, ICU admission, mechanical ventilation, high APACHE II score, leukopenia, leukocytosis, cancer, specific pneumococcal serotypes, inappropriate initial antimicrobial therapy, and infection with a non-susceptible strain.3,7,21–24 In our work, in-hospital mortality was associated to three independent factors: age ≥65 years old, CAP PIRO score>3, and a platelet count<100,000/μL. These findings came as no surprise because older age is a recognized risk factor, a CAP PIRO score>3 represents a known high risk group of patients affected by community-acquired pneumonia,10 and thrombocytopenia has been occasionally described as a risk factor in bacteremic patients.25 All together, they reflect severely ill patients with predisposing conditions.
The only independent factor associated with intensive or intermediate care unit admission was C-reactive protein. This parameter shows high values in patients with CAP and bacteremia compared to those without this condition.6 Increased values are also seen in subjects with CAP and sepsis6 and in CAP caused by Legionella pneumophila, Enterobacteriaceae or S. pneumoniae, but not in cases associated to viruses or atypical agents.6 Despite C-reactive protein not be included in predictive scales it could be a good, simple biomarker of severity in patients with bacteremic streptococcal pneumonia that deserve more studies. However, it will probably not be of universally valid because of the lower values reported in patients with severe viral infections, such as those with pandemic influenza A H1N1.26
Three severity scores were analyzed in this work but they did no differ statistically according to the AUC observed in ROC analysis. A similar AUC for the PIRO score reported in patients admitted to ICU with CAP was observed in another study (0.86 vs. 0.88) as well as for the APACHE II score (0.77 vs. 0.75).10 Using the same cut-off (>3) for the CAP PIRO score applied in the above mentioned reference gave a similar sensitivity and specificity (85% vs. 86% and, 77% vs. 79%). These predictive scores appear to have suboptimal test properties as witnessed by their limited sensitivities and specificities, and low positive predictive values and likelihood ratios. Other reports from Chile have obtained even lower AUC in the case of CURB-65 and other predictive scores.27 These limitations suggest that these scores can be an option while managing these patients once admitted, and clinicians can prefer to use a predictive scale that is easier to calculate. The CURB-65 score is currently used in Chile in a national guideline on CAP to discriminate and select patients for appropriate ambulatory treatment or admission. In this case, this predictive score is applied on an ambulatory basis and not after admission.
Results of one randomized trial and meta-analysis suggest that corticoids may be of benefit in severely-ill patients with CAP.28,29 We found that during the first 72h almost 40% of the patients received corticoid therapy, but no reduction of in-hospital mortality was observed. According to a post hoc analysis of one randomized trial, the benefit on mortality appears to be restricted to a specific group of patients characterized by high cytokine and reduced cortisol levels.30
S. pneumoniae serotype frequencies have been scarcely described among adult patients in Latin America.31,32 This information is highly necessary to evaluate the preventive potential of immunization programs, and changes or emergence of specific serotypes, especially after vaccine introduction.9,33 In a comprehensive surveillance study conducted between 2000 and 2005 in several Latin America countries, 5657 pneumococcal strains from adult patients were serotyped.31 Eleven of the 13 most frequent serotypes reported in that study, were detected in our series. This match is not surprising since 33.5% of the strains of that surveillance study came from Chile. According to our findings the serotype coverage of the 23-valent polysaccharide vaccine and the 13-valent conjugated vaccine would be 72%, and 50%, respectively. These figures are rather disappointing because one of the most accepted polyvalent polysaccharide vaccine benefits is precisely the protection against invasive forms of disease, but is serotype specific.34,35 This limitation is enhanced by the low immunization record detected in this work, either for influenza, as a predisposing condition, or for S. pneumoniae. A national immunization program for the latter was launched in 2009 in Chile for the elderly population, but coverage still remains low with less than 6% of the targeted population vaccinated by 2011. Five serotypes have been associated to a higher risk of death (3, 6A, 6B, 9N, 19F) and three to a lower risk (1, 7F, 8).23,36,37 Twenty-one isolates in our study matched these serotypes but we did not find association with an adverse outcome (data not shown). Of interest, one patient with liver cirrhosis had a recurrent infection with the same serotype (serotype 5) after three years. Although infrequent, this phenomenon has been described before.38
Low prevalence of antimicrobial resistance among pneumococcal isolates from adult patients in Chile has been a stable finding.39 Less than 1% of CSF or non-CSF isolates have shown resistance to penicillin or third generation cephalosporins. Consequently, inappropriate initial antibiotic therapy did not have a contributory influence in the outcome of our patients, as has been demonstrated in other reports.7
In conclusion, our series of adult patients admitted with bacteremic pneumococcal pneumonia was characterized by an older age and a high prevalence of comorbidities, a severe clinical condition, frequent hospitalization in critical care beds (50%), and mechanical ventilation (30%). Some of them had an abrupt onset (13%) and previous immunization against influenza or S. pneumoniae was remarkably rare. Multilobar infiltrates were rather common (30%) and in-hospital mortality was high (33%). Cases were not associated to a few predominant serotypes, and no beta-lactam resistance was observed. Multivariate analysis identified C-reactive protein>150mg/L as the only factor associated to critical care hospitalization. In addition, death risk was linked to three independent factors: a severity CAP PIRO score>3, age≥65 years, and platelet count<100,000/μL. The predictive yield of APACHE II, CURB-65 and CAP PIRO scores was not statistically different, but demonstrated suboptimal sensitivities and specificities or positive likelihood ratios. Also, a seasonal pattern was observed, with higher incidence of cases during mid-fall and early winter. Finally, a non-significant decreasing trend of bacteremic pneumococcal pneumonia cases was observed through the study years that paralleled a reduction in mortality due to pneumonia in elderly patients in Chile.
Conflict of interestThe authors declare no conflicts of interest.