Infections caused by Streptococcus pneumoniae (pneumococcus) still represent a challenge for health systems around the world.
ObjectiveThe objective of this study was to assess microbiological and clinical aspects in hospitalized patients with invasive pneumococcus disease between 1998 and 2013.
Materials and methodsThis was a retrospective study that analyzed the results of pneumococcus identification, serotyping, and susceptibility testing found in the Adolfo Lutz Institute databank. Personal variables, medical history and clinical outcome of patients admitted with invasive pneumococcal disease were analyzed. These were obtained from records of a public teaching hospital – Hospital das Clínicas Faculdade de Medicina Ribeirão Preto.
ResultsThe sample comprised 332 patients. Patient age ranged from less than one month to 89 years old (mean 20.3 years) and the sample was predominately male. Pneumonia (67.8%) was the most common disease, accounting for 18.2% of deaths. Serotypes 14, 1, 3, 9V, 6B, 6A, 23F, 19A, 18C, 19F, 12F, and 4 were the most common (75.3%). Most patients, or 67.5%, were cured without any complication (success), 6.9% had some type of sequela (failure), and 25.6% died (failure). In the case of deaths due to meningitis, strains of fully penicillin resistant pneumococcus were isolated. Furthermore, 68.2% of patients who died presented some type of comorbidity. The 60 and older age group presented the most significant association (Odds Ratio=4.2), with outcome failure regardless of the presence of comorbidity. Serotype 18C was the most significant risk factor both in raw analysis (Odds Ratio=3.8) and when adjusted for comorbidity (Odds Ratio=5.0) or age (Odds Ratio=5.4). The same occurred with serotype 12F (respectively, Odds Ratio=5.1, Odds Ratio=5.0, and Odds Ratio=4.7)
ConclusionThe present findings highlight the importance of IPD among young adults and older adults. In the era of conjugate vaccines, monitoring serotypes in different age groups is essential to assess the impact and adequacy of immunization.
Even with the possibility of immunoprevention of several serotypes, the World Health Organization has indicated and estimated 1.6 million deaths per year due to pneumococcus disease. The data demonstrate greater prevalence in developing countries and 0.7–1 million children less than five years of age.1 In countries with immunization for H. influenzae serotype b, pneumococcus has become the most common cause of invasive disease.2 Based on data from the Hospital Information System (HIS), Novaes, Sartori, and Soárez3 found that there were 34,217 hospitalizations due to pneumococcal disease in the Brazilian Unified Health System (SUS) between 2006 and 2014. This figure represented 0.1% of the total of admissions, of which 64.8% were pneumococcal pneumonia. A study on the clinical and epidemiological profile of patients with pneumonia acquired in the community observed among individuals under the age of 60 an elevated percentage of the disease, in addition to worsened clinical outcomes. Lethality was low (4.9%) and concentrated among the youth. In Brazil, these patients are usually hospitalized in order to ensure access to medication and clinical follow-up, especially in the case of older adults and individuals with underlying chronic diseases.4 According to the Brazilian Ministry of Health,5 between 2000 and 2008 pneumococcal meningitis represented 11% of bacterial meningitis recorded in the Notifiable Diseases Information System (SINAN), with an average of 1218 cases per year and 30% lethality. In order to minimize the impact of pneumococcal disease, in 2009 the National Agency of Health Surveillance (ANIVSA) approved the use of conjugate pneumococcal polysaccharide vaccine (PVC) with 10 specific serotypes (PVC10) in children under the age of two. Thus, Brazil was one of the first countries to include the VPC10 in the immunization schedule of the National Immunization Program (PNI).6,7 The objective of this study was to analyze clinical and microbiological aspects of people hospitalized with IPD between 1998 and 2013, and therefore evaluate the outcome of cases in relation to the serogroups.
Materials and methodsThis was a retrospective cross-sectional study that analyzed records from 1998 to 2013. These records contained the results of 332 isolated pneumococcal strains in patients with invasive pneumococcus disease (IPD) hospitalized in the Ribeirão Preto School of Medicine Teaching Hospital–University of São Paulo (HCRP-USP), Brazil. The pneumococcus samples included in this study were isolated in the HCRP laboratory and sent to Adolfo Lutz Institute in Ribeirão Preto (IAL-RP) in order to confirm the species, and then forwarded to the National Laboratory of Public Health (Adolfo Lutz Institute in São Paulo) for serotyping and to determine the antimicrobial susceptibility profile. Toward this end, microbiological information was obtained from the IAL-RP archives and the clinical aspects of patients retrieved from the surveillance system of HCRP-USP epidemiological center.
Analysis and inference of resultsThe information gathered was saved on an MS Excel XP spreadsheet and then exported to SPSS (Statistical Package for the Social Sciences), version 19.0 for statistical analyses: descriptive analysis through absolute and relative frequencies and then means, standard deviations, medians, and minimum and maximum values were calculated. Possible associations between outcomes: success (cure with no complications) or failure (cure with complications or death). The studied variables (gender, age, presence of comorbidity, and the most common serotypes) were analyzed using chi-square test and univariate (raw OR) and multiple (adjusted OR) logistic regression. Level of significance was set at p≤0.05. This was a retrospective study, with limitations associated with the nature of information in quantitative and qualitative terms. The present study was approved by the human subject research ethics committee, protocol CAAE: 26759714.80000.5393.
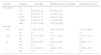
ResultsAmong the 332 patients with IPD, age ranged from less than a month to 89 years old, with mean age of 20.3 (standard deviation=25.5 years, median 4 years), with a predominance of male patients (61.1%). The most common clinical diagnoses were pneumonia (67.8%), followed by meningitis and bacteremia (22.9%). After 2010, there was a significant reduction in the number of pneumococcus cases sent for identification (Table 1). Forty-two different serotypes of S. pneumoniae were identified, of which 75.3%, in decreasing order, were serotypes 14, 1, 3, 9V, 6A, 6B, 23F, 19A, 18C, 19F, 12F, and 4 with at least 10 isolates. Furthermore, there was a progressive increase of serotypes 3 and 6A, which were present in the 13-valent conjugate pneucomococcal vaccine, and serotypes 12F and 18C (non-vaccine). There was a reduction in serotypes 14, 1, and 23F included in VPC10 (Table 2). The prevalence of the serogroups varied according to age of the patients. Serogroup 14 was the most common in children younger than five years of age, and serogroup 3 was the most common in the age range greater than five years old (Table 3). In children younger than five years of age, in the period prior to the VPC10, there was a prevalence of serogroups 14 (46.4%), 1 (8.6%), 19A (6.6%), 6A (6.0%), and 9V (4.6%) in hospitalized patients. After 2010, serogroups 6A (23.1%), 14 (23.1%) and 3 (23.1%) were the prevailing serogroups in this age range. Treatment was successful among 67.5% of patients, with no complications, while 32.5% of cases resulted in treatment failure, with 25.6% deaths and 6.9% with some type of sequelae. Of the IPD cases that resulted in death, 68.2% (58/85) occurred in patients older than five years of age. Among patients less than five years old, 46% (17/37) of the deaths were associated with meningitis and 9.8% (4/41) with pneumonia. Of the 85 deaths (25.6%) due to IPD, meningitis was involved in 43.5% of the cases, with a 48.7% lethality rate (37/76) across all age groups. Of all meningitis related deaths, 29.7% (11/37) occurred in children younger than one year, and 27.0% (10/37) in patients with ages between 20 and 59, representing a lethality rate of 21.2% (11/52) for patients less than one year old, and 12.0% (10/83) among those aged 20 through 59. Pneumonia accounted for 48.2% of deaths, with a lethality rate of 18.2% (41/225). Of all deaths due to pneumonia, 21.2% (18/85) occurred in the 20–59 age group, and 4% in patients less than five years of age. Regarding the antibiotic susceptibility profile of the strains, 9.4% (n=8) of deaths due to meningitis were caused by penicillin-resistant S. pneumoniae (penicillin MIC ≥0.125μg/mL), represented by serotypes 14 (3.5%, n=3), 23F (2.4%, n=2), 19A (1.2%, n=1), 6B (1.2%, n=1), and 9V (1.2%, n=1). A total of 68.2% of patients presented comorbidities, the most common being HIV/AIDS (27.6%), alcoholism, and cancer (17.2%). Serotypes 12F, 14, 18C, 9V, 18A, 19A, and 23F accounted for 65% of all deaths due to meningitis, while serotypes 3, 14, 9V, 6B, 23F, and 19F were involved in 63.4% of deaths due to pneumonia. There was no association between patient gender and outcome in terms of success or failure (p=0.568). Regarding the presence of complications and death related to patient age, we found that the older the patient, the higher the percentage of sequelae and death (p<0.001). Furthermore, the presence of comorbidity was associated with outcome failure (p=0.002). Among patients with no comorbidities, 22.9% presented treatment failure, whereas among those with comorbidities the failure rate was 38.8% (Table 4). Our analysis of each serotype with at least 10 isolates in relation to clinical outcomes revealed no significant difference for serotypes 3, 9V, 6B, 6A, 23F, 19F, 19A, and 4. Serotype 18C had the highest failure rate when compared with non-18C serotypes (63.6×31.5%; p=0.025). The same was true for serotype 12F (70.0×31.4%; p=0.010). Serotype 14 presented the lowest failure rate when compared to all other serotypes (17.1×37.6%; p=0.001), as well as serotype 1 (8.0×34.5%; p=0.006). Univariate analysis demonstrated that patients less than 20 years were at the greatest risk. After adjusting for the presence of comorbidities, age or serotype were significantly associated with type of outcome only in those with 60 years or more (OR=4.2) (Table 4). Furthermore, serotypes 14 (p=0.001) and 1 (p=0.006) were less likely to result in failure (OR=0.3 and OR=0.2, respectively), regardless of the presence of comorbidities (OR=0.4 and OR=0.2, respectively) (Table 5). However, after adjusting for age, this association was no longer significant (Table 4). In the present study, serotypes 18C (p=0.025) and 12F (p=0.010) resulted in the greatest failure rates when compared to the other serotypes. The presence of 18C represented a signfiicant risk factor, both in unadjusted analysis (OR=3.8) and after adjusting for comorbidity (OR=5.0) or age (OR=5.4), as did serotype 12F (OR=5.1, OR=5.0, and OR=4.7, respectively) (Table 6).
Demographic characteristics and clinical diagnosis of patients with IPD registered in the HCRP Databank, 1998–2013.
| Variable | Category | No. | % |
|---|---|---|---|
| Gender | Male | 203 | 61.1 |
| Female | 126 | 38.0 | |
| Unknown | 3 | 0.9 | |
| Age group (years) | <1 | 52 | 15.7 |
| 1–2 | 59 | 17.8 | |
| 2–5 | 57 | 17.2 | |
| Subtotal <5 | 168 | 50.7 | |
| 5–20 | 40 | 12.0 | |
| 20–60 | 83 | 25.0 | |
| ≥60 | 39 | 11.7 | |
| Subtotal ≥5 | 162 | 48.7 | |
| Unknown | 2 | 0.6 | |
| Period | 1998–2002 | 81 | 24.4 |
| 2003–2010 | 205 | 61.7 | |
| 2011–2013 | 46 | 13.9 | |
| Disease | Pneumonia | 225 | 67.8 |
| Meningitis | 76 | 22.9 | |
| Bacteremia/septicemia | 26 | 7.8 | |
| Others | 5 | 1.5 | |
| Total | 332 | 100.0 |
IPD, invasive pneumococcal disease; HCRP, Ribeirão Preto Teaching Hospital.
Serotypes of S. pneumoniae most often isolated from IPD patients registered in the HCRP Databank, 1998–2013.
| Serotypes | Periods | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 1998–2002 | 2003–2010 | 2011–2013 | No. | % | ||||
| No. | %a | No. | %a | No. | %a | |||
| 14 | 31 | 38.3 | 51 | 25.0 | 3 | 6.4 | 85 | 25.6 |
| 1 | 7 | 8.6 | 17 | 8.3 | 0 | 0.0 | 24 | 7.2 |
| 3 | 2 | 2.5 | 16 | 7.8 | 5 | 10.6 | 23 | 6.9 |
| 9V | 5 | 6.2 | 9 | 4.4 | 2 | 4.3 | 16 | 4.8 |
| 6A | 3 | 3.7 | 10 | 4.9 | 3 | 6.4 | 16 | 4.8 |
| 6B | 3 | 3.7 | 10 | 4.9 | 2 | 4.3 | 15 | 4.5 |
| 23F | 3 | 3.7 | 12 | 5.9 | 0 | 0.0 | 15 | 4.5 |
| 19A | 6 | 7.4 | 5 | 2.5 | 1 | 2.1 | 12 | 3.6 |
| 18C | 5 | 6.2 | 4 | 2.0 | 2 | 4.3 | 11 | 3.3 |
| 19F | 0 | 0.0 | 10 | 4.9 | 1 | 2.1 | 11 | 3.3 |
| 12F | 0 | 0.0 | 4 | 2.0 | 6 | 12.8 | 10 | 3.0 |
| 4 | 1 | 1.2 | 8 | 3.9 | 1 | 2.1 | 10 | 3.0 |
| Others | 15 | 18.5 | 48 | 23.5 | 21 | 44.7 | 84 | 25.3 |
| Total | 81 | 100.0 | 204 | 100.0 | 47 | 100.0 | 332 | 100.0 |
Serogroups of S. pneumoniae most commonly isolated from patients with invasive pneumococcal disease who were assisted at the Regional Health Care Network 13, according to the age of the patients, from 1998 to 2013.
| Serotypes | Age range (years) | Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1–2 | 2–5 | Subtotal <5 | 5–20 | 20–60 | ≥60 | Subtotal ≥5 | No. | % | |||||||||
| No. | %a | No. | %a | No. | % | No. | %a | No. | %a | No. | %a | No. | %a | No. | % | |||
| 14 | 20 | 38.5 | 26 | 44.1 | 29 | 50.9 | 75 | 44.6 | 4 | 10.0 | 6 | 7.2 | 0 | 0.0 | 10 | 6.2 | 85 | 25.8 |
| 1 | 1 | 1.9 | 2 | 3.4 | 10 | 17.5 | 13 | 7.7 | 8 | 20.0 | 2 | 2.4 | 1 | 2.6 | 11 | 6.8 | 24 | 7.3 |
| 3 | 3 | 5.8 | 5 | 8.5 | 2 | 3.5 | 10 | 6.0 | 1 | 2.5 | 6 | 7.2 | 6 | 15.4 | 13 | 8.0 | 23 | 7.0 |
| 9V | 2 | 3.8 | 4 | 6.8 | 1 | 1.8 | 7 | 4.2 | 2 | 5.0 | 4 | 4.8 | 3 | 7.7 | 9 | 5.5 | 16 | 4.8 |
| 6A | 6 | 11.5 | 2 | 3.4 | 5 | 8.8 | 13 | 7.7 | 2 | 5.0 | 1 | 1.2 | 0 | 0.0 | 3 | 1.8 | 16 | 4.8 |
| 6B | 4 | 7.7 | 3 | 5.1 | 0 | 0.0 | 7 | 4.2 | 0 | 0.0 | 5 | 6.0 | 3 | 7.7 | 8 | 4.9 | 15 | 4.5 |
| 23F | 2 | 3.8 | 2 | 3.4 | 0 | 0.0 | 4 | 2.4 | 3 | 7.5 | 6 | 7.2 | 2 | 5.1 | 11 | 6.8 | 15 | 4.5 |
| 19A | 6 | 11.5 | 3 | 5.1 | 1 | 1.7 | 10 | 5.9 | 0 | 0.0 | 1 | 1.2 | 1 | 2.6 | 2 | 1.2 | 12 | 3.6 |
| 18C | 2 | 3.8 | 2 | 3.4 | 3 | 5.3 | 7 | 4.2 | 1 | 2.5 | 1 | 1.2 | 2 | 5.1 | 4 | 2.5 | 11 | 3.3 |
| 19F | 0 | 0.0 | 0 | 0.0 | 2 | 3.5 | 2 | 1.2 | 3 | 7.5 | 4 | 4.8 | 2 | 5.1 | 9 | 5.5 | 11 | 3.3 |
| 12F | 1 | 1.9 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 2 | 5.0 | 6 | 7.2 | 1 | 2.6 | 9 | 5.5 | 10 | 3.0 |
| 4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 5.0 | 6 | 7.2 | 2 | 5.1 | 10 | 6.2 | 10 | 3.0 |
| Others | 5 | 9.6 | 10 | 16.9 | 4 | 7.0 | 19 | 11.3 | 12 | 30.0 | 35 | 42.2 | 16 | 41.0 | 64 | 39.5 | 83 | 25.1 |
| Total | 52 | 100.0 | 59 | 100.0 | 57 | 100.0 | 168 | 100.0 | 40 | 100.0 | 83 | 100.0 | 39 | 100.0 | 162 | 100.0 | 330 | 100.0 |
Demographic characteristics and presence of comorbidity among IPD patients registered in the HCRP Databank by case outcome, 1998–2013.
| Variable | Category | Cure no sequela | Cure with sequela/death | Total | p-value | |||
|---|---|---|---|---|---|---|---|---|
| No. | %a | No. | %a | No. | % | |||
| Gender | Male | 134 | 66.0 | 69 | 34.0 | 203 | 61.1 | 0.568 |
| Female | 87 | 69.0 | 39 | 31.0 | 126 | 38.0 | ||
| Unknown | 3 | 0.9 | 0 | 0.0 | 3 | 0.9 | ||
| Age (years) | <1 | 38 | 73.1 | 14 | 26.9 | 52 | 15.7 | <0.001 |
| 1–2 | 44 | 74.6 | 15 | 25.4 | 59 | 17.8 | ||
| 2–5 | 48 | 84.2 | 9 | 15.8 | 57 | 17.2 | ||
| Subtotal <5 | 130 | 77.4 | 38 | 22.6 | 168 | 50.6 | ||
| 5–20 | 35 | 87.5 | 5 | 12.5 | 40 | 12.0 | ||
| 20–60 | 44 | 53.0 | 39 | 47.0 | 83 | 25.0 | ||
| ≥60 | 15 | 38.5 | 24 | 61.5 | 39 | 11.7 | ||
| Subtotal ≥5 | 94 | 58.0 | 68 | 42.0 | 162 | 48.7 | ||
| Unknown | 1 | 0.3 | 1 | 0.3 | 2 | 0.6 | ||
| Comorbidity | No | 108 | 77.1 | 32 | 22.9 | 140 | 42.2 | 0.002 |
| Yes | 112 | 61.2 | 71 | 38.8 | 183 | 55.1 | ||
| Unknown | 4 | 44.4 | 5 | 55.6 | 9 | 2.7 | ||
| Total | 332 | 100.0 | ||||||
Most common serotypes of S. pneumoniae in comparison to other serotypes isolated from IPD patients registered in the HCRP Databank by case outcome, 1998–2013.
| Serotypes | Cure no sequela | Cure with sequela/death | Total | p-value | |||
|---|---|---|---|---|---|---|---|
| No. | %a | No. | %a | No. | % | ||
| 14 | 68 | 82.9 | 14 | 17.1 | 82 | 100.0 | 0.001 |
| Non-14 | 156 | 62.4 | 94 | 37.6 | 250 | 100.0 | |
| 1 | 23 | 92.0 | 2 | 8.0 | 25 | 100.0 | 0.006 |
| Non-1 | 201 | 65.5 | 106 | 34.5 | 307 | 100.0 | |
| 3 | 14 | 58.3 | 10 | 41.7 | 24 | 100.0 | 0.321 |
| Non-3 | 210 | 68.2 | 98 | 31.8 | 308 | 100.0 | |
| 9V | 9 | 52.9 | 8 | 47.1 | 17 | 100.0 | 0.189 |
| Non-9V | 215 | 68.3 | 100 | 31.7 | 315 | 100.0 | |
| 6B | 9 | 56.2 | 7 | 43.8 | 16 | 100.0 | 0.326 |
| Non-6B | 215 | 68.0 | 101 | 32.0 | 316 | 100.0 | |
| 6A | 12 | 80.0 | 3 | 20.0 | 15 | 100.0 | 0.289 |
| Non-6A | 212 | 66.9 | 105 | 33.1 | 317 | 100.0 | |
| 23F | 11 | 73.3 | 4 | 26.7 | 15 | 100.0 | 0.620 |
| Non-23F | 213 | 67.2 | 104 | 32.8 | 317 | 100.0 | |
| 19F | 5 | 45.5 | 6 | 54.5 | 11 | 100.0 | 0.113 |
| Non-19F | 219 | 68.2 | 102 | 31.8 | 321 | 100.0 | |
| 19A | 9 | 64.3 | 5 | 35.7 | 14 | 100.0 | 0.795 |
| Non-19A | 215 | 67.6 | 103 | 32.4 | 318 | 100.0 | |
| 18C | 4 | 36.4 | 7 | 63.6 | 11 | 100.0 | 0.025 |
| Non-18C | 220 | 68.5 | 101 | 31.5 | 321 | 100.0 | |
| 12F | 3 | 30.0 | 7 | 70.0 | 10 | 100.0 | 0.010 |
| Non-12F | 221 | 68.6 | 101 | 31.4 | 322 | 100.0 | |
| 4 | 7 | 70.0 | 3 | 30.0 | 10 | 100.0 | 0.862 |
| Non-4 | 217 | 67.4 | 105 | 32.6 | 322 | 100.0 | |
Adjusted analysis of factors associated with outcome failure among patients with IPD registered in the HCRP Databank, 1998–2013.
| Variable | Category | Raw OR | OR adjusted for comorbidity | OR adjusted for age |
|---|---|---|---|---|
| Age (years) | <1 | 1 | 1 | |
| 1–2 | 0.9 [0.4; 2.2] | 0.9 [0.4; 2.2] | ||
| 2–5 | 0.5 [0.2; 1.3] | 0.5 [0.2; 1.4] | ||
| 5–20 | 0.4 [0.2; 1.2] | 0.4 [0.1; 1.2] | ||
| 20–60 | 2.4 [1.1; 5.1] | 2.1 [0.9; 4.8] | ||
| ≥60 | 4.3 [1.8; 10.6] | 4.2 [1.6; 10.9] | ||
| Serotype | ||||
| 18C | Yes | 3.8 [1.1; 13.3] | 5.0 [1.4; 18.0] | 5.4 [1.4; 20.9] |
| No | 1.0 | 1.0 | 1.0 | |
| 12F | Yes | 5.1 [1.3; 20.1] | 5.0 [1.2; 20.1] | 4.7 [1.1; 21.3] |
| No | 1.0 | 1.0 | 1.0 | |
| 14 | Yes | 0.3 [0.2; 0.6] | 0.4 [0.2; 0.8] | 0.5 [0.3; 1.1] |
| No | 1.0 | 1.0 | 1.0 | |
| 1 | Yes | 0.2 [0.1; 0.7] | 0.2 [0.1; 0.9] | 0.3 [0.1; 1.2] |
| No | 1.0 | 1.0 | 1.0 | |
IPD, invasive pneumococcal disease; HCRP, Ribeirão Preto Teaching Hospital.
There is a vast amount of literature with information on the characteristics of Streptococcus pneumoniae strains that cause invasive disease, especially related to the development of new vaccines. In countries in which the conjugate pneumococcal vaccine was included in the immunization schedule, there was a sharp decline in hospitalization rates due to pneumonia, especially among children under the age of three years.8 In developing countries, approximately 15 million children with pneumonia were hospitalized every year9; this IPD stands out due to its high lethality.10 The reduction in pneumococcus samples sent for identification after 2010 was compatible with the findings of Afonso and collaborators,7 who found that one year after the implementation of VPC10 in Brazil there was a drop in the number of hospitalized children with pneumonia in the municipalities of Belo Horizonte, Recife, and Curitiba. In Brazil, data from the public health information system (DATASUS) indicate pneumonia as the leading cause of hospital admissions, with the greatest occurrence at extremes of age, affecting approximately 2.1 million Brazilians per year.11 Considering all age groups, the incidence density of pneumococcal meningitis in the state of São Paulo during the period of this study ranged between 0.9 and 1.5/100,000 inhabitants.12 It was observed that 68.2% of deaths among all of the analyzed IPDs occurred in patients under the age of five. The global lethality rate of IPDs in industrialized countries ranges from 15% to 20% among adults, and from 30% to 40% among patients with comorbidities, or older adults whose immunological function is compromised.13 In European countries, Navarro-Torné et al.14 reported a 15.9% death rate due to pneumococcal meningitis, with an association between penicillin-resistant of S. pneumoniae and increased risk of death. In the present study, we observed a 43.5% rate of meningitis, with 9.4% of deaths involving penicillin-resistant strains of S. pneumoniae. In the city of Salvador15 and in the Amazon state,16 the death rate due to pneumococcal meningitis surpassed 37.0% and 32.4%, respectively, in all age groups. Based on SINAN data from 2001 to 2010, Grando et al.17 found that the annual mean death rate due to pneumococcal meningitis in Brazil was 33.6% and 31.3% in children less than five years old and less than one year old, respectively. In addition to presenting high mortality rates, meningitis is also a serious concern due to the association with severe neurological complications, as well as high treatment costs. Edmond and collaborators,18 attributed serious sequelae to pneumococcal meningitis, such as hydrocephalus, cognitive and motor deficits, hearing loss, behavioral problems, and learning disorders. In the present investigation, the rate of sequelae was 6.9%. Kyaw et al.19 indicated that, in the United States, pneumococcal disease in adults was associated with a higher incidence among patients with HIV/AIDS, alcoholism, heart disease, respiratory diseases, and diabetes mellitus. In accordance with the results obtained in the present study, 68.2% of patients who died presented some type of comorbidity, primarily HIV/AIDS. In Brazil, data on lethality of respiratory diseases are scarce. However, according to Cashat-Cruz et al.,20 death from acute respiratory diseases is among the most common causes of death in the Americas, accounting for approximately 40% of cases. This study demonstrated that pneumonia-related mortality was high (48.2%), especially among adults, and only 4% of cases involved patients younger than five. In Spain, mortality related to pneumococcal pneumonia varied from 7.4% to 13% dependending on the patient's age.21 In Portugal, pneumonia is the second leading cause of death among patients hospitalized with respiratory diseases, with a 22% lethality rate.22 In the present study IPD cases were more often caused by serotypes 18C and 12F, representing a significant risk factor for failure. Several authors suggest that the different serotypes possess have different potential to cause invasive disease, indicating an association between fatal outcomes for pneumococcal disease and capsular serotypes of pneumococcus.14,23–27 However, there was a variation in serotypes more frequently involved in fatal cases, namely serotypes 3, 6B, 9N, 19F, and 7F described by these authors as the most invasive and involved in unfavorable outcomes; on the other hand serotype 1 presented the lowest risk of death. Furthermore, these authors also found serotypes 3, 6B, 18C, and 19F to be among the most frequently involved in patient deaths. In turn, serotypes 1, 7F, 8, 6A, and 15 showed no relation with fatal cases. In this context, information about the risk associated with different serotypes, in terms of different outcomes of IPDs, allows us to estimate the expectation before new vaccine formulas; for example the inclusion of serotype 3 may not help reducing the morbidity associated with IPD.24 However, the results of this study underscore the influence of age as a risk factor for acquiring IPD, corroborating the findings of Navarro-Torné,14 who indicated that individuals older than 65 had a higher tendency to develop serious pneumococcal diseases associated with a greater rate of morbidity and mortality than children less than five years old. As observed in the present study, the higher risk related to older adults is not dependent on the presence of comorbidities, as was found in a study by Santos et al.,28 who demonstrated that the presence of comorbidities did not seem to influence the final outcome of IPDs. Due to impaired splenic function, immunosenescence, and changes in the respiratory tract,29 older adults are more susceptible to pneumococcal disease. In Brazil, the Geriatric Vaccination Guideline30 recommends the 23-valent pneumococcal polysaccharide vaccine (PPV23) and the 13-valent pneumococcal conjugate vaccine (PVC13) for patients above the age of 60 or those who have specific chronic underlying diseases. However, the 13-valent vaccine is not available for older adults through the national immunization program, and is offered only by private vaccination clinics. In turn, the PPV23 is administered to institutionalized older adults and, when medically indicated, to those who are part of the high-risk group for pneumococcal infection. In this sense, after assessing the cost of PPV23 in adults aged 60 or older, Tonolio Neto and colleagues31 found that, in Brazil, incorporating this vaccine to the national immunization program would be very cost-effective given the costs of hospitalization and absenteeism. However, despite the evidence pointing to the effectiveness of the VPP23 against IPDs,32 its effectiveness in preventing pneumonia in adults and high-risk patients, for whom it is recommended,33 as well as its preventive action in immunosuppressed patients, is still questioned.34 In this context, a study conducted in Germany concluded that in comparison with the VPP23, the VPC13 was better indicated to reduce the burden of pneumococcal disease, even economically.35 Another study conduced by Polish researchers found that older adults above the age of 65, regardless of risk for contracting pneumococcal infection, should be vaccinated with VPC13.36 In 2010, based on the data about pneumococcal pneumonia in Spain, Luja et al.37 suggested that the new VPC13 would result in a sharp decline in mortality among the older adult population. Ardanuy and collaborators38 emphasized the importance of administering the conjugate vaccine to adults, suggesting that the VPC13 should be used in Spain for that age group. Corroborating the data of a double-blind randomized trial conducted in Holland, which found a reduction in pneumococcal pneumonia among older adults after immunization with VPC13,39 Picazo et al.40 found that VPC13 does not lead to the phenomenon of serotype replacement as observed with the 7-valent pneumococcal vaccine. In light of the above and the high incidence and mortality rate of IPDs among adults and especially older adults, we emphasize the need to review vaccination recommendations and programs for these age groups, especially considering the increase in serotypes 3 and 6A observed in the present study. However, there is controversy surrounding the effectiveness of these vaccines in adults, as well as the most appropriate choice for this population.41 In this context, O’Brien42 highlighted the importance of immunogenicity studies to determine the effectiveness of conjugate vaccines against pneumonia in adults, especially in the context of co-infection with the H1N1 influenza virus.
ConclusionThe pneumococcus isolates among patients hospitalized with invasive pneumococcal disease presented variability in terms of circulating serotypes. This emphasizes the importance of ongoing monitoring of invasive strains in different manifestations of pneumococcal disease, especially considering the progressive increase in serotypes 3 and 6A present in the 13-valent conjugate pneumococcal vaccine, and serotypes 12F and 18C, which are non-vaccine serotypes. Serotypes 18C and 12F were significant risk factors both in unadjusted and adjusted analysis for comorbidity and age. Furthermore, regarding case outcomes, 25.6% resulted in death, especially among patients with meningitis. Patients aged 60 years or older presented the most statistically significant association (OR=4.2) with outcome failure, regardless of the presence of comorbidities. In light of this information we recognize the importance of conducting surveillance studies that can underpin the process of reviewing and making decisions regarding public policies and immunization programs for all adults and for older adults in particular. In addition, in the age of conjugate vaccines, information about the distribution of serotypes among different age groups is essential to assess the impact and adequacy of immunization programs.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for funding this study.
Part of a doctoral thesis “Serotypes and antimicrobial resistance profile of Streptococcus pneumoniae: clinical implications for invasive disease and the national immunization program” (1998–2013).