Three decades after HIV recognition and its association with AIDS development, many advances have emerged – especially related to prevention and treatment. Undoubtedly, the development of Highly Active Antiretroviral Therapy (HAART) dramatically changed the future of the syndrome that we know today. In the present study, we evaluate the impact of Highly Active Antiretroviral Therapy on macrophage function and its relevance to HIV pathogenesis.
MethodsPBMCs were isolated from blood samples and monocytes (CD14+ cells) were purified. Monocyte-Derived Macrophages (MDMs) were activated on classical (MGM-CSF+IFN-γ) or alternative (MIL-4+IL13) patterns using human recombinant cytokines for six days. After this period, Monocyte-Derived Macrophages were stimulated with TLR2/Dectin-1 or TLR4 agonists and we evaluated the influence of HIV-1 infection and Highly Active Antiretroviral Therapy on the release of cytokines/chemokines by macrophages.
ResultsThe data were obtained using Monocyte-Derived Macrophages derived from HIV naïve or from patients on regular Highly Active Antiretroviral Therapy. Classically Monocyte-Derived Macrophages obtained from HIV-1 infected patients on Highly Active Antiretroviral Therapy released higher levels of IL-6 and IL-12 even without PAMPs stimuli when compared to control group. On the other hand, alternative Monocyte-Derived Macrophages derived from HIV-1 infected patients on Highly Active Antiretroviral Therapy released lower levels of IL-6, IL-10, TNF-α, IP-10 and RANTES after LPS stimuli when compared to control group. Furthermore, healthy individuals have a complex network of cytokines/chemokines released by Monocyte-Derived Macrophages after PAMP stimuli, which was deeply affected in MDMs obtained from naïve HIV-1 infected patients and only partially restored in MDMs derived from HIV-1 infected patients even on regular Highly Active Antiretroviral Therapy.
ConclusionOur therapy protocols were not effective in restoring the functional alterations induced by HIV, especially those found on macrophages. These findings indicate that we still need to develop new approaches and improve the current therapy protocols, focusing on the reestablishment of cellular functions and prevention/treatment of opportunistic infections.
Since the discovery that HIV was the cause of the AIDS and the establishment of this condition as the main cause of death associated with infectious diseases, recent data estimate that over 35 million people around the world are living with HIV.1 During the last three decades, there have been many advances in the understanding of viral cycle replication and pathogenesis-related mechanisms. As a result several antiretroviral drugs were developed. Specifically, the combined Highly Active Antiretroviral Therapy (HAART) resulted in the reduction of deaths related to AIDS, helped controlling the viral spread and increased the quality of life and the life expectancy of HIV-1 infected patients.2–4 However, many challenges still hamper the development of new and more effective therapeutic approaches to functional cure.
Classically, the immune dysfunction observed during HIV infection is directly associated with an intense reduction of CD4+ T cells in peripheral blood and other tissues including intestinal mucosa. In recent years several studies have demonstrated the contribution of innate immunity to viral pathogenesis and AIDS development.5–7 This paper will focus on the role of macrophages, but other authors have explored the relevance of dendritic cells and neutrophils on HIV pathogenesis.8–10
Monocyte-Derived Macrophages (MDMs) play a central role in the immune response, orchestrating the development of innate and adaptive immunity to pathogens, and further, these MDMs can be infected chronically by HIV.11 Besides remaining viable, HIV infected MDMs can be differently activated and develop several functional impairments, with reduced phagocytic and intracellular killing activity, thus allowing for the occurrence of opportunistic infections.12–14
In the recent years, Chihara and colleagues have demonstrated that HIV-1 proteins including gp120, Tat, and Nef induce macrophage polarization towards the classical pathway13 and, according to Cassol and colleagues, enable macrophage support for increased viral replication.15 Currently, few papers have evaluated the role of antiretroviral drugs on functional immune recovery after the onset of regular therapy protocols. In our study we evaluated the influence of HIV-1 infection on cytokine/chemokine release by classically (MGM-CSF+IFN-γ) or alternatively (MIL-4+IL13) activated MDMs after PAMPs stimuli on MDMs derived from treatment-naïve patients or from those on regular HAART.
Material and methodsCasuistic and selection criteriaEight treatment-naïve HIV-1 infected patients on early stage of disease were recruited for this study. In addition, 15 HIV-1 infected patients on regular HAART (HIV-1+HAART) for at least six months and 15 healthy blood donors were included as controls (Table 1). Informed consent was obtained from all participants included in the study. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The present study was approved by the Clinical Hospital of FMRP/USP Ethics Committee (#9817/2012).
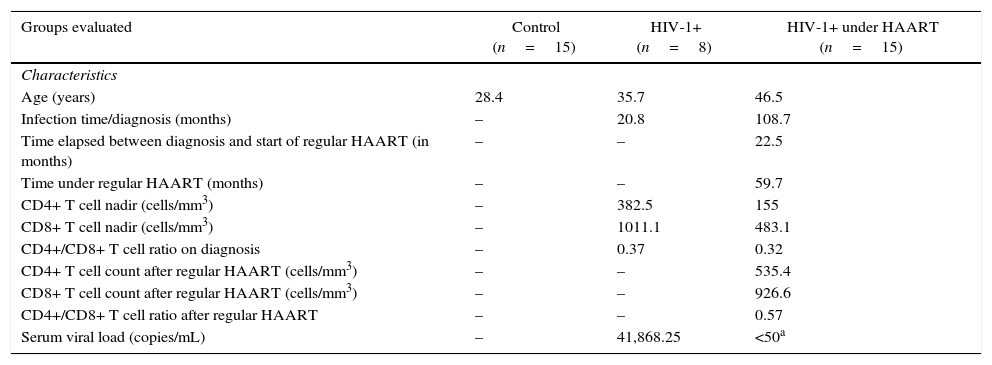
General characteristics of patients included in the study.
| Groups evaluated | Control (n=15) | HIV-1+ (n=8) | HIV-1+ under HAART (n=15) |
|---|---|---|---|
| Characteristics | |||
| Age (years) | 28.4 | 35.7 | 46.5 |
| Infection time/diagnosis (months) | – | 20.8 | 108.7 |
| Time elapsed between diagnosis and start of regular HAART (in months) | – | – | 22.5 |
| Time under regular HAART (months) | – | – | 59.7 |
| CD4+ T cell nadir (cells/mm3) | – | 382.5 | 155 |
| CD8+ T cell nadir (cells/mm3) | – | 1011.1 | 483.1 |
| CD4+/CD8+ T cell ratio on diagnosis | – | 0.37 | 0.32 |
| CD4+ T cell count after regular HAART (cells/mm3) | – | – | 535.4 |
| CD8+ T cell count after regular HAART (cells/mm3) | – | – | 926.6 |
| CD4+/CD8+ T cell ratio after regular HAART | – | – | 0.57 |
| Serum viral load (copies/mL) | – | 41,868.25 | <50a |
HAART, Highly Active Anti-Retroviral Therapy.
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque™ PLUS (GE Healthcare), following the manufacturer's instructions. The obtained PBMCs were quantified and the monocytes (CD14+ cells) were purified using positive selection with magnetic system microbeads (Miltenyi Biotec). After elution, the CD14+ cells were cultured in 48-well plates with RPMI 1640 medium containing 1μL/mL gentamicin, supplemented with 10% of FBS (Gibco Laboratories) and incubated at 37°C, 5% CO2. To induce classical (MGM-CSF+IFN-γ) or alternative (MIL-4+IL13) MDMs activation, CD14+ cells were cultured for six days in medium containing GM-CSF (20ng/mL; R&D Systems) and IFN-γ (10ng/mL; Millipore) or IL-4 and IL-13 (50ng/mL each; R&D Systems), respectively. Resting MDMs (M0) were cultured with standard medium without supplementation of cytokines. Every two days in culture, fresh medium containing the respective conditions (M0, MGM-CSF+IFN-γ or MIL-4+IL13) was added to keep cell viability.
Quantification of cytokines and chemokines on supernatant after PAMPs stimuliAfter the respective activation, MDMs were stimulated with 10ng/mL of LPS (Sigma–Aldrich) or 100μg/mL of β-glucan extracted from Saccharomyces cerevisiae (Calbiochem, Merck Millipore) during 24h. After this period, the supernatant was collected and nine cytokines and chemokines released (IL-1β, IL-6, IL-10, IL-12, IFN-α2, TFN-α, IP-10, MCP-1 and RANTES) were quantified using a multiplex assay (HCYTOMAG-60K, Milliplex® kit, Merck Millipore, Germany) with Luminex® Magpix™ technology (Austin, TX, USA). The assay was performed following the manufacturer's instructions. The cytokines/chemokines concentrations were calculated by Milliplex Analyst 5.1® software using a standard curve with cubic spline fitting (log scale).
Statistical analysisAll data are presented as median with interquartile range and the classical statistical analysis was performed using Kruskal–Wallis test followed by Dunn's test between all columns pairs. For correlation analysis was used Spearman's two-tailed test and the networks of correlations of cytokines/chemokines released was built using Cytoscape software (v.3.2.0), classifying the interactions according to the following ratio: negative (r<0); weak (r<0.36); moderate (0.36<r<0.67); or strong (r>0.67). In both analyses, p≤0.05 was considered significant.
ResultsHIV-1 patients under regular use of HAART present more pronounced impairment of cytokines/chemokines released from MDMs after PAMPs stimuliThe present data show that classical MDMs derived from both treatment-naïve HIV-1 infected patients and HIV-1+HAART groups expressed very similar levels of cytokines and chemokines after PAMPs stimuli. In spite of that, we observed an increase of IL-6 and IL-12 released by unstimulated MDMs derived from HIV-1+HAART patients compared to control patients (Fig. S1). On the other hand, alternatively activated MDMs obtained from the analyzed groups showed a lower level of IL-6, IL-10, TNF-α, IP-10, and RANTES released by HIV-1+HAART after LPS stimuli compared to control patients (Fig. S2).
MDMs obtained from treatment-naïve HIV-1 infected patients present several impairments in cytokines/chemokines expression after PAMPs stimuli and these alterations were not restored with antiretroviral therapyTo fully explore the impact of HAART on the development of the MDM-dependent immune response, we examined the expression of cytokines/chemokines from classically or alternatively activated MDMs derived from treatment-naïve HIV-1 infected patients, HIV-1+HAART or control groups after PAMP stimuli.
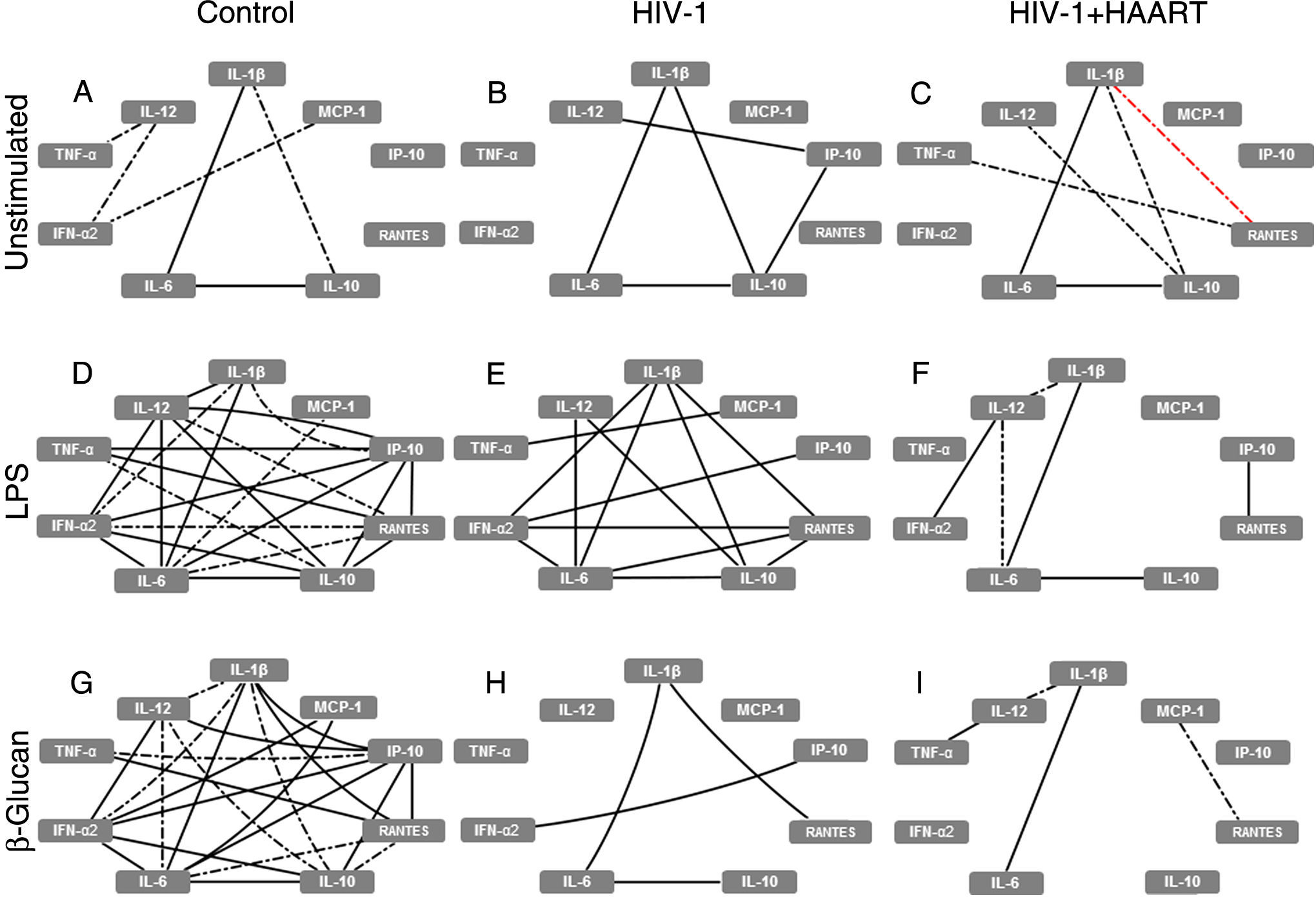
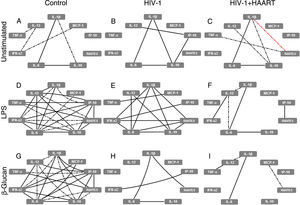
At baseline (without PAMP stimuli), classical MDMs derived from control patients expressed a few positive correlations, especially strong between IL-1β↔IL-6↔IL-10 (forming the ‘elementary triangle’) and moderate between IL-1β↔IL-10 and TNF-α↔IL-12↔IFN-α2↔MCP-1 (Fig. 1A). However, these interactions changed when we analyzed the MDM cytokine expression patterns obtained from treatment-naïve HIV-1 infected patients. In these patients the elementary triangle is preserved, intensifying the correlation between IL-1β↔IL-10 and forming a new strong correlation amongst IL-12↔IP-10↔IL-10 (Fig. 1B), suggesting a ‘primed’ proinflammatory profile of MDMs obtained from treatment-naïve HIV-1 infected patients. In HIV-1+HAART patients without PAMP stimuli, the elementary triangle was similar to the control group, with an increase of new moderate positive correlations (IL-10↔IL-12; TNF-α↔RANTES) and, interestingly, a negative (IL-1β↔RANTES) correlation (Fig. 1C). Nevertheless, the main changes observed amongst the groups with respect to classical MDMs’ function occurred after PAMP stimuli. The complex network observed after LPS stimuli on MDMs derived from the control group (Fig. 1D) showed decreased interactions among cytokines from treatment-naïve HIV-1 infected patients, particularly affecting IL-6, IL-12, TNF-α and IP-10 (Fig. 1E). Decreased cytokine correlations were strongly accentuated in MDMs obtained from HIV-1+HAART patients; in contrast, only six of 23 interactions obtained from the control group were preserved (Fig. 1F). Similarly, after recognition of β-glucan, classical MDMs derived from the control group formed a complex correlation network (Fig. 1G) that was progressively losing the ‘elementary triangle’ and other important correlations seen in the treatment-naïve HIV-1 infected and HIV-1+HAART groups–preserving only four interactions vs 23 obtained in the control group (Fig. 1H and I).
HIV-1 infection induces damage on cytokine and chemokine network formed by classical MDMs that are not restored even under HAART. The analysis of correlation between the cytokines and chemokines released by unstimulated, LPS- or β-glucan-stimulated classical MDMs was performed using Spearman's two-tailed test. The network was built using Cytoscape software (v3.2.0). Solid lines indicate strong correlations and dash-dot lines are representative of moderate correlations. All the black lines represent positive correlations between the targets and the red lines are representative of negative correlations.
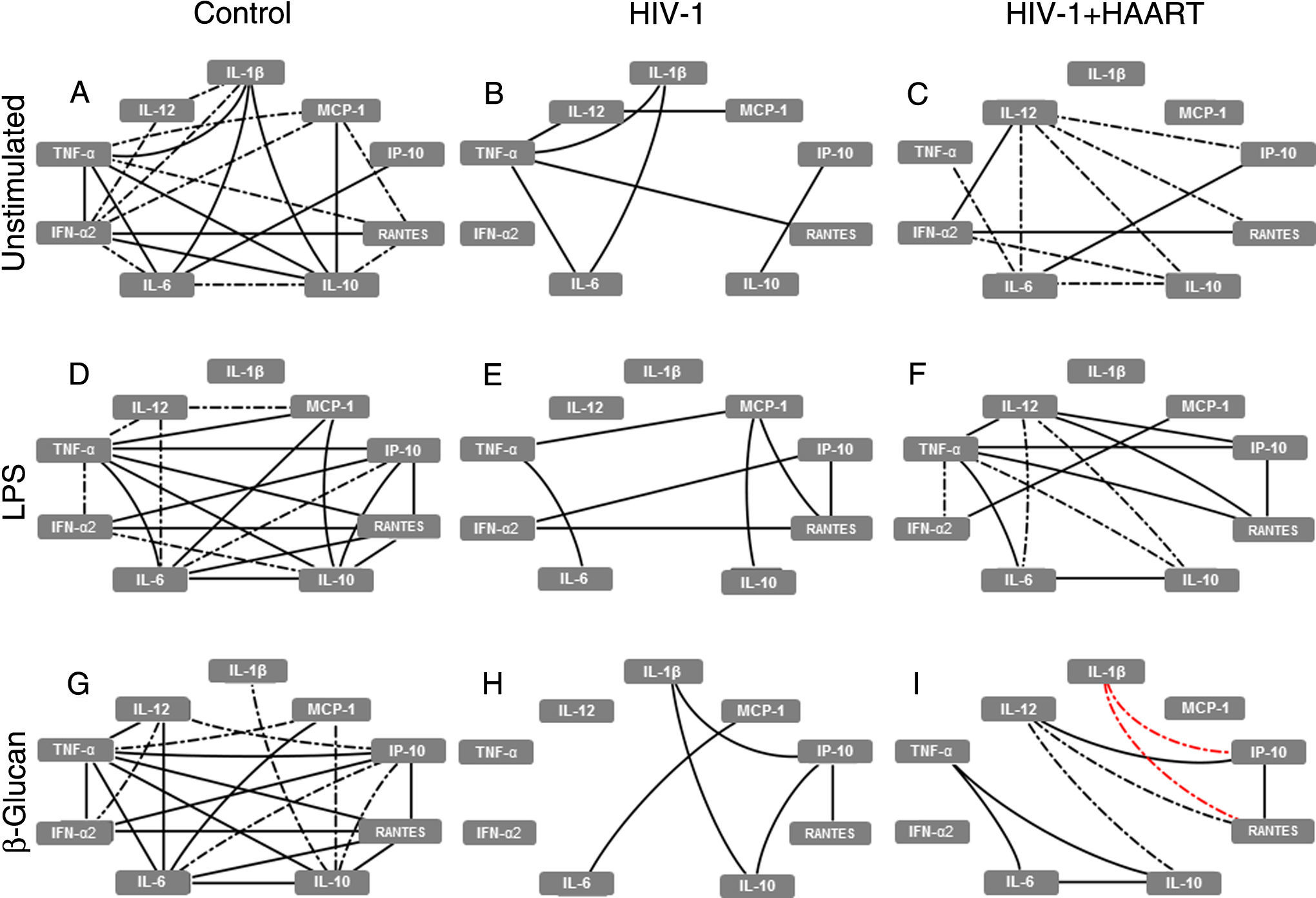
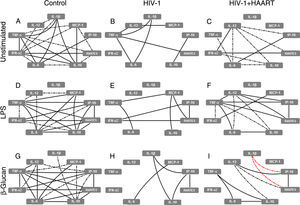
In parallel, the baseline analysis of alternative MDM network correlations revealed that the complex correlations (including the elementary triangle) seen in the control group was not present in treatment-naïve HIV-1 infected patients (only seven of 20 previous interactions remained) and was only partially restored in HIV-1+HAART patients (with 10 of 20 correlations observed), reducing the IL-10 and TNF-α correlations and increasing moderate interactions with IL-12 (Fig. 2A–C). After stimuli with LPS and β-glucan, a similar pattern of correlation was observed amongst the groups, represented by complex interactions in the control group (mainly based on TNF-α; Fig. 2D and G) and reduced numbers and intensity of correlations found in MDMs from treatment-naïve HIV-1 infected patients (Fig. 2E and H). Additionally, the analysis of supernatants obtained from HIV-1+HAART patients after LPS and β-glucan stimuli resulted in correlations similar to those found in the control group as well as two new negative correlations among RANTES↔IL-1β↔IP-10 after β-glucan stimuli (Fig. 2F and I).
HIV-1 infection induces damage on cytokine and chemokine networks formed by alternative MDMs that are not restored even under HAART. The analysis of correlation between the cytokines and chemokines released by unstimulated, LPS- or β-glucan-stimulated alternative MDMs was performed using Spearman's two-tailed test. The network was built using Cytoscape software (v3.2.0). Solid lines indicate strong correlations and dash-dot lines are representative of moderate correlations. All the black lines represent positive correlation between the targets and the red lines are representative of negative correlations.
Classically, the term “macrophage” refers to a set of terminally differentiated cells with low proliferation capacity that have different names according to their localization.16 Despite the common name, macrophages do not represent a homogeneous population and can originate from two distinct embryological sources. The first wave of macrophages is generated during early embryogenesis and is derived from Yolk Sac progenitors, giving rise to physiological tissue macrophages (named microglia, Langerhans cells, Kupffer cells and alveolar macrophages) with the marked ability to self-renew in the periphery.17–19 On the other hand, the second wave of macrophages is derived from Hematopoietic Stem Cells (HSCs) that become circulating blood monocytes, which after migration to peripheral tissues differentiate into MDMs.20
Under physiological conditions, MDMs display a full spectrum of activation in different tissues, reflecting the microenvironment milieu in which they find themselves.21 The recognition of PAMPs (e.g., LPS) and/or proinflammatory cytokines induces the classical macrophage (M1) activation pathway, related to Th1 CD4+ T cell activation, while the recognition of glucocorticoids, immune complexes, or others cytokines (e.g., IL-4, IL-10 and IL-13) induces the alternative macrophage (M2) activation pathway, related to Th2 CD4+ T cell activation.22,23
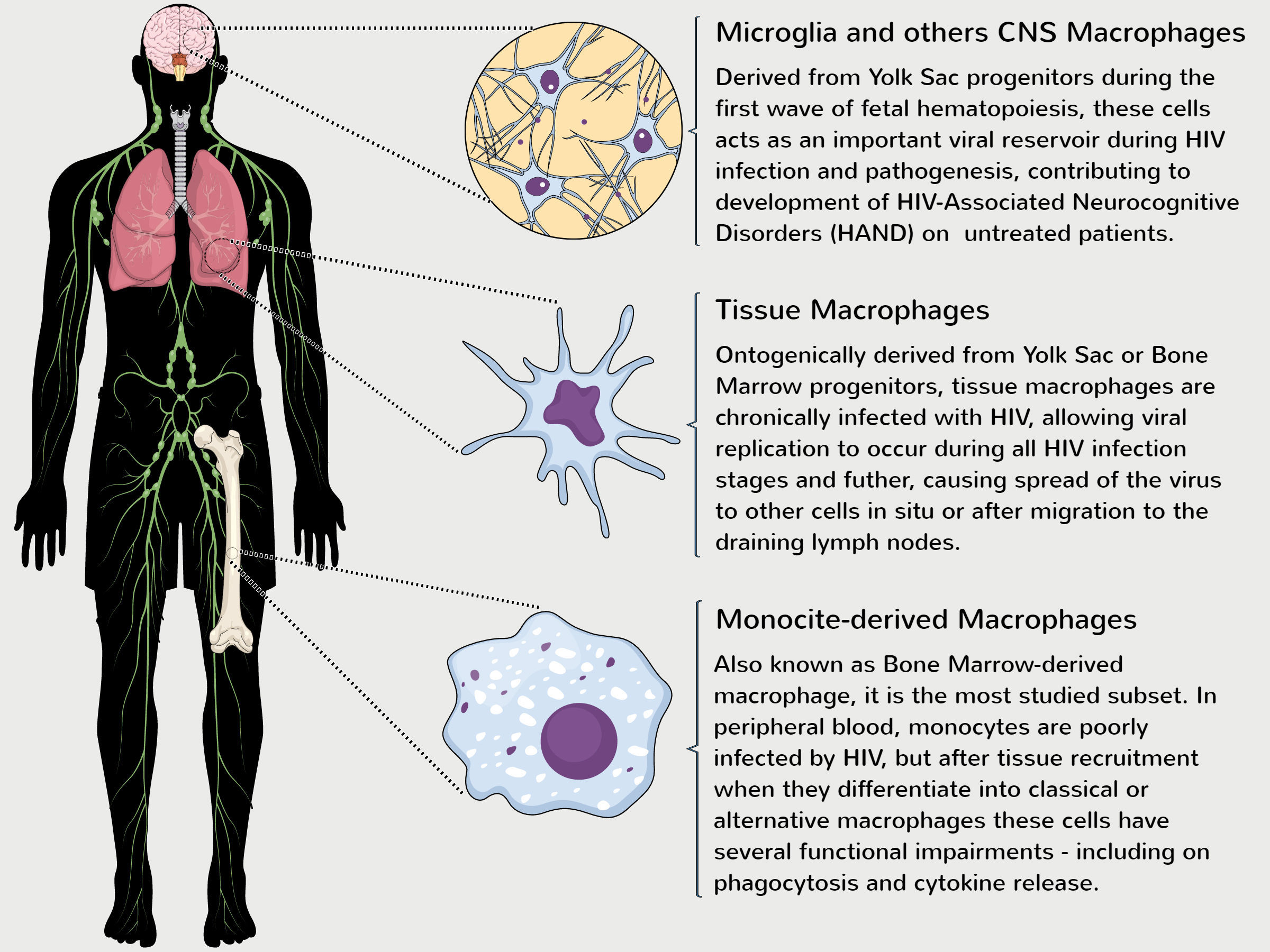
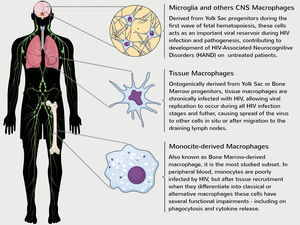
Although embryological distinct, both macrophage subsets can productively be infected and contribute to HIV pathogenesis during all stages of infection.24 The first evidence of HIV-infected macrophages were published in the 1980s,25,26 but it was not until recently this field has been better explored. In this context, our knowledge of macrophage function is based mainly on studies of MDMs and new studies are critically needed to close the large gap of knowledge relating to YSDMs function during HIV infection (Fig. 3).
Role of distinct macrophage populations on development of HIV-1 pathogenesis. Despite being ontogenically distinct, YSDMs and MDMs have a critical role in the maintenance of viral replication, reflected in the development of chronic HIV-1 pathogenesis. MDMs are derived from the terminal differentiation of myeloid bone marrow progenitor cells and several studies have demonstrated that MDMs obtained from HIV-1 patients have functional impairments in vitro.12–14 Although monocytes are poorly infected in the bloodstream, HIV-1 can infect directly CD34+ progenitor cells and induce functional impairments during hematopoiesis.29–31 Some tissue macrophages are derived from monocyte differentiation of peripheral tissues or from Yolk Sac progenitors seeded during the early steps of embryogenesis.17–19 Both of these subsets can be chronically infected and cause the spread of virus to other cells. In parallel, microglia and other CNS (as perivascular and meningeal) macrophages are directly derived from YS progenitors and contribute to development of neuronal death and HAND found mainly on untreated HIV patients.24,33
Over the years, different authors demonstrated that only a few monocytes found on peripheral blood are infected by HIV-1,27,28 while others studies demonstrated that the virus can directly infect bone marrow progenitors.29–31 Despite this paradox, after migration to peripheral tissues, monocytes differentiate into macrophages and, even though expressing several factors restricting viral replication,32 these MDMs are chronically infected and act as an important viral reservoir, allowing HIV replication and spread to other cells (including CD4+ T cells) during the normal time course of infection.11,33 This viral replication strategy is critical to HIV pathogenesis and gives these macrophages a ‘Trojan horse’ status, that can be directly associated with the development of comorbidities, such as immunosenescence and inflammatory/cardiovascular diseases, and the progression to AIDS.34,35
Herein we have shown the increase of IL-6 and IL-12 released from classical MDMs derived from HIV-1+HAART. This may be associated to prolonged time of infection and higher baseline activation of macrophages induced by microbial translocation from gut to systemic circulation, a common phenomenon found in HIV infected patients.36 As consequence of these events, a massive release of proinflammatory cytokines (also known as ‘cytokine storm’) takes place in plasma and the increase of baseline levels of cellular activation status. Interestingly, alternatively activated MDMs obtained from HIV-1+HAART patients release lower levels of IL-6, IL-10, TNF-α, IP-10, and RANTES compared to control patients after LPS stimuli.
Based on these results, we hypothesized that the reduction of proinflammatory cytokines can be a secondary damage induced by the chronicity of HIV infection and establishment of viral reservoirs, which could be reprogramming the macrophage function, or even could be induced by regular antiretroviral therapy. Despite the relevance of HAART in controlling viral load and restoring CD4+ T cells count and CD4+/CD8+ T cell ratio in HIV infected patients, regular HAART would impair macrophage function after PAMP stimuli in these patients, which may be related to global reduction of proinflammatory status found in HIV-infected patients. These hypotheses are supported by the good therapeutic adherence and the late infection stage on the onset of therapy of HIV-1+HAART patients (Table 1), suggesting that these failures are due to prolonged time of HIV infection and also by the treatment per se.
Taken together, our data reveal that alternatively activated MDMs are more adversely affected after HIV-1 infection than classically activated MDMs and the antiretroviral therapy did not completely restore the MDMs functionality after PAMP stimuli. These results are consistent with previous reports demonstrating that alternatively activated macrophages are more affected after contact with HIV-1 proteins. Furthermore, this contact induces the reprogramming of alternative towards classical MDMs pattern, and this imbalance may be associated with the spread of viral infection13 and the failure of T cell-dependent immune responses that further contribute to development of AIDS.37
Undoubtedly, the development of antiretroviral drugs in the 1990s and the improvement of therapeutic protocols have changed the course of HIV-1 infection in the last 20 years.38,39 Currently, the FDA has approved 26 antiretrovirals for treating HIV infection, including combined therapies that target different steps of viral entry or the replication cycle.40 Usually after a few weeks on HAART, the viral load and the inflammatory mediators decrease drastically in plasma, often falling to undetectable levels and stabilizing the CD4+ T cells counts. However, even under continuous therapy, patients maintain HIV-1 replication in different tissue reservoirs that are responsible for the long term persistence of viral infection.41 Based on the above positive results, the WHO now recommends antiretroviral therapies be made available to all patients diagnosed and living with HIV.42 Relatedly, our group and others recently have demonstrated that the adoption of HAART works to effectively reduce the viral load and the inflammation biomarkers in plasma,43–45 reducing both the effects of the systemic inflammatory disease and the comorbidities/mortality associated with HIV infection.46,47
Despite these therapeutic successes, no currently used drug is able to restore the MDM functional alterations induced by HIV or directly strengthen the immune response of infected patients. These findings indicate that we still need to develop new approaches and improve our current therapeutic protocols, focusing on reestablishment of cellular functions and prevention/treatment of opportunistic infections.
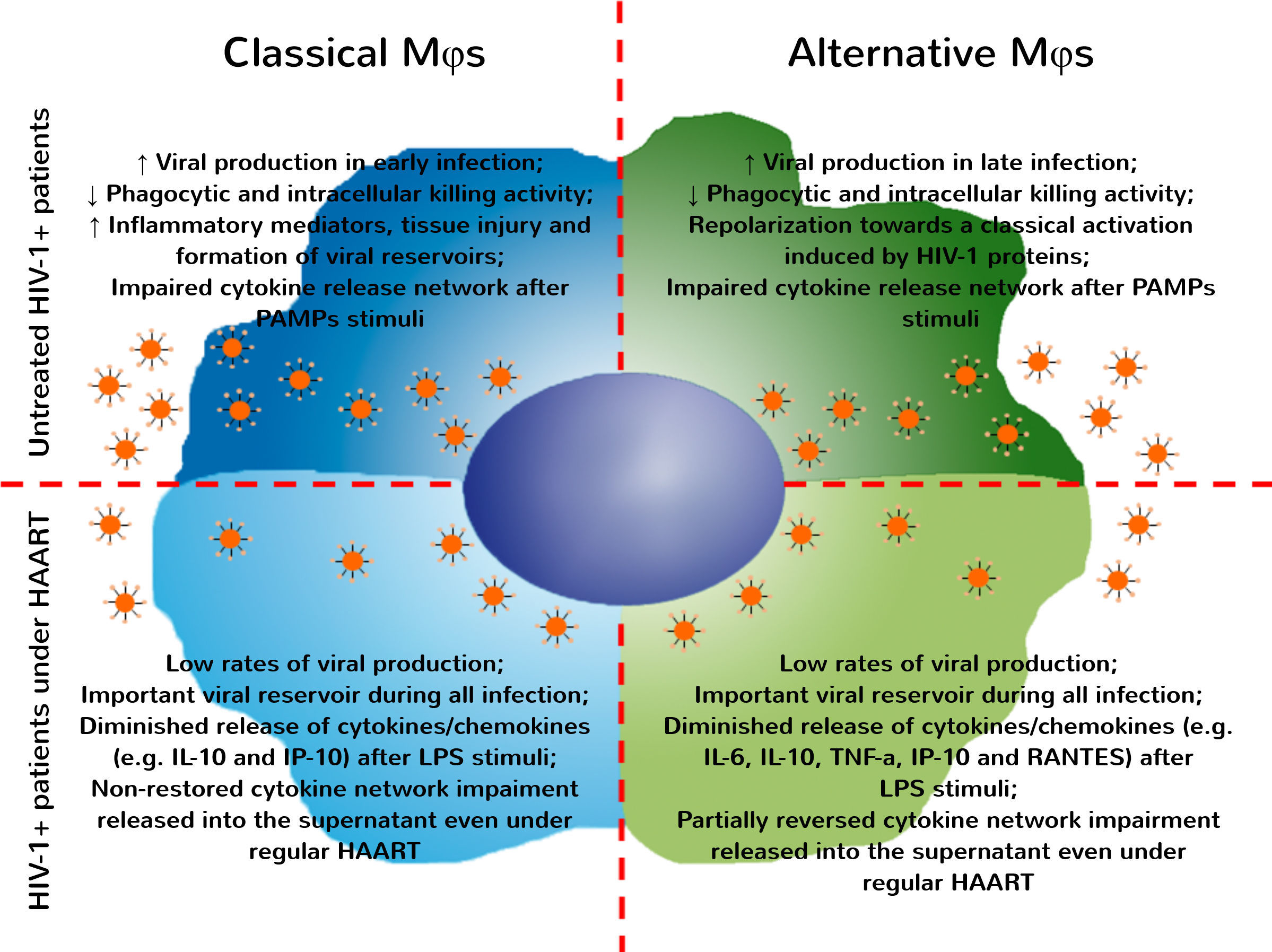
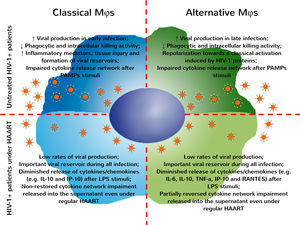
Therefore, these observations suggest an important effect of antiretroviral drugs on MDMs function, even without PAMP stimuli in vitro. Under regular HAART, HIV-1+ patients exhibited a pronounced reduction of inflammatory mediators (including sCD14, sCD163 and TF) associated with microbial translocation and development of comorbidities associated with infection,48–50 thus improving the life expectancy of these patients. Recently, our group showed that treatment with second line therapy protocols do not restore the immune activation status, despite the reduction of viral load found in plasma samples.43 In the present paper, we examined MDMs function during HIV-1 infection and demonstrated an important reduction in cytokine/chemokine released by classically and alternatively activated MDMs derived from HIV-1 infected patients at baseline and after recognition of PAMP stimuli. After all, it its unquestionable the relevance of HAART during HIV infection and its benefits to avoid AIDS development. Several recent studies evidenced that the beginning of combined antiretroviral therapy even in newly diagnosed patients has a great epidemiological relevance and is being encouraged by WHO.51 Nonetheless, we cannot miss the point that the current HAART protocols do not restore the impairments found in macrophages or in other immune cells obtained from HIV-infected patients, and these functional changes may be associated to the development of other non-AIDS related comorbidities (Fig. 4). In Brazil, since the year of 2014, all HIV infected patients are entitled to start treatment irrespective of comorbidities or CD4+ T cell counting and viral load level.52 Therefore, in the data showed here, considering the CD4+ T cell nadir, CD4+/CD8+ T cell ratio on diagnosis, and the time elapsed since diagnosis and start of regular therapy, the HIV-treated patients were in a late stage of infection compared to the treatment-naïve HIV-1 infected group. In fact, the advanced stages of HIV infection may induce several impairments on immune response that could be reflected in our cytokine data. Another point to consider is the immunosenescence. It is well documented that HIV infection is related to premature immunosenescence53,54 and the prolonged time of infection could accelerate the occurrence of functional alterations in macrophages. Thus, it is crucial that new adjuvant therapies should be developed, considering the aim at restoring cellular function while eliminating viral reservoirs.
HIV-1 infection induces several impairments on classical and alternative MDMs that were not fully restored even under regular HAART. After infection by HIV-1, MDMs activation is influenced by interaction with viral proteins and responsible for sustaining viral replication over different stages of infection. Classical and alternatively activated MDMs present functional impairments – as reduction in phagocytic and intracellular killing activity and the general profile of cytokines/chemokine released after recognition of antigenic stimuli. Even under regular HAART, MDMs obtained from HIV-1+ patients are chronically infected and represent an important viral reservoir. The functional changes found in these cells are only partially restored, impairing the development of appropriate immune responses against pathogens, allowing the occurrence of opportunistic infections.
The authors declare no conflicts of interest.
The funding for this work was provided by the São Paulo Research Foundation (FAPESP, grants #2011/12199-0 and #2012/02799-3) and CAPES. The authors are grateful to Hemocentro de Ribeirão Preto and Hospital das Clínicas de Ribeirão Preto – FMRP/USP. The authors thank Dr. Judith Connett for her thorough reading of the manuscript.