Chikungunya is an arthropod-borne virus transmitted by Aedes mosquito bites. A viral mutation has allowed Aedes albopictus to become the preferred vector extending the geographic spread of the condition. The virus causes an acute febrile illness occasionally followed by a chronic rheumatic condition causing severe impairment. The diagnosis is usually confirmed with serology. No specific treatment is currently available. This article reviews the condition with emphasis on his dissemination in the Americas.
Chikungunya virus has disseminated widely and autochthonous cases have already been reported in the Americas. Although the disease tends to be self-limited, a crippling chronic condition with severe joint compromise can affect patients for weeks to months. Health practitioners need to be acquainted with the manifestations, diagnostic methods and treatment options for this formerly “exotic” condition.
AgentThe Chikungunya virus (CHIKV) is an arthropod-borne virus that belongs to the family Togaviridae, genus Alphavirus. Its genome is composed by a single stranded positive polarity RNA molecule. The genome codifies four non-structural proteins (NS P 1-4) and three structural proteins (C, E1 and C2). The virus gets destroyed by desiccation and by temperatures above 58°C.1 The alphavirus genus includes about 29 species, seven of these viruses can causes joint disorders in humans including CHIKV, O’nyong-nyong (Central Africa), Ross River and Barmah Forest (Australia and the Pacific), Semliki Forest (Africa), Sindbis (Africa, Asia, Australia and Europe), and Mayaro (South America and the French Guyana).2
There are three lineages of CHIKV with distinctive genotypic and antigenic characteristics. The virus isolated during the 2004–2006 epidemics in the Indian Ocean belongs to a distinct set within the largest phylogenetic group East/Central/South African (ECSA). However the Asian lineage is the one currently ravaging the Americas. The other group is the West African lineage.3
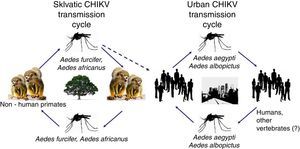
CHIKV persists in nature using two cycles: a sylvatic cycle affecting primates and mosquitoes and an urban cycle affecting humans and mosquitoes (Fig. 1).
Virus replication occurs following these steps:
- -
Early replication of RNA into mRNA and translation of early regulatory proteins
- -
Late replication of the RNA into mRNA and translation of late structural proteins
- -
Assembly of structural proteins and single stranded positive RNA, and virion maturation.4
Although there is an ample range of Aedes species that transmit the disease in Africa5; in Asia and in the Indian Ocean the main vectors of CHIKV are Aedes aegypti and Aedes albopictus. A. albopictus has a wider geographical distribution, and can survive in both rural and urban environments. Mosquito eggs are quite resistant to dry seasons. A. albopictus also has a relatively long life, lasting 4–8 weeks and has a flying range of 400–600m.6 All these capabilities have allowed A. albopictus to become an important vector not only of CHIKV, but also of dengue and other arbovirosis. A comparison between A. aegypti and A. albopictus is presented in Table 1.
Comparison between Aedes aegypti and Aedes albopictus.
| Vector | Aedes aegypti | Aedes albopictus |
|---|---|---|
| Local distribution | Predominantly an urban vector, breeds close to households, can bite indoor or outdoors | Predominantly a rural vector, breeds far from households, mostly an outdoor biter |
| Global distribution | Narrower global distribution | Wider global distribution |
| Tendency to bite humans | Occasional | Aggressive |
| Preferred source of blood meals | Prefers humans | Affects humans and a variety of vertebrates |
| Nickname | Yellow fever mosquito | Asian tiger mosquito |
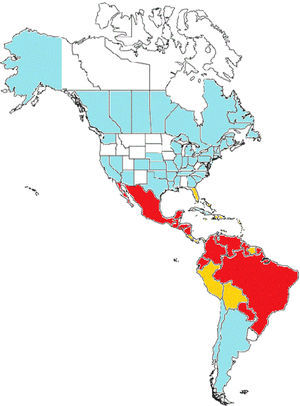
In Brazil, an extensive DDT campaign eradicated A. aegypti from the country in the 1940–50s, however the vector was reintroduced in 1970 and become widespread again. Since 1986 it has been considered endemic in several major Brazilian cities. A. albopictus invaded Brazil in the 1980s and a recent survey has detected it in at least 59% of the Brazilian municipalities and in 24 of the 27 federal units7,8A. aegypti affects predominantly tropical areas of Brazil (North, North-East and Central regions) and is more widespread, whereas A. albopictus is more common in the cooler Southern areas of the country. Both vectors combined put 99% of the population of Brazil at risk of acquiring CHIKV.9Fig. 2 shows the geographic distribution of Chikungunya virus in the Americas.
Chikungunya in the Americas. In red: countries with endemic transmission with more than 1000 cases reported. In orange: Countries with endemic transmission with more than 1000 cases reported. In blue: Countries or States with imported cases only. Without color: Countries or States with no transmission reported.
In the United States, A. aegypti has been established for more than 300 years and since 1985 the Southeast of the United States has been invaded by A. albopictus, with a range extending from South Florida to Illinois.10
The adaptation of CHIKV to A. albopictus is a relatively recent event. During the outbreak in the Indian Ocean in 2005–2006 the virus has acquired a mutation at residue 226 of the membrane fusion glycoprotein-1, which allows it to infest A. albopictus. This mutation is likely the cause of the wide spread of the disease.11
PathogenesisCHIKV is transmitted predominantly by female mosquito bites. Alternatively the disease can be transmitted vertically from mother to fetus or theoretically by blood transfusion (although no cases have been reported so far).
After the inoculation, the virus invades endothelial cells and subcutaneous fibroblasts and replicates in a limited fashion. Circulating blood cells may be refractory to invasion. New viruses are transported to local lymph nodes where they further replicate. A significant viremia which can reach up to 108copies/mL then ensues.12 In the initial phases there is a massive infection of monocyte-derived macrophages which act as “Trojan horses” and transport the virus into target organs including muscle, joints, the liver and the brain.13
The innate immune response is activated by the virus via pattern recognition receptors. This triggers the production of type I interferon and activates interferon-stimulated genes which encode more than 300 proteins, with crucial roles in the host defense. In vitro studies have shown that CHIKV can be highly suppressed when interferon α/β is added to cells prior to infection.14
Flow cytometry showing CD8+ T lymphocyte response in the early stages of the disease and a CD4+ T lymphocyte-mediated response in the later stages, as well as production of several pro-inflammatory cytokines are evidence of a subsequent adaptive immunity reaction.15 There is heterogeneity in the cytokines expressed, reflecting the time of the illness in which they are measured and the different genetic backgrounds of the individuals affected. The persistence of a local reservoir of infected monocytes in the joints may potentially explain chronic arthritis in a subset of patients. Patients with chronic joint disease may have high levels of interleukin 6 and granulocyte macrophage colony-stimulating factor, but not of tumor necrosis factor (TNF) or IL-1b (a pattern seen in other inflammatory arthritides). Chronically affected patients also have normal levels of hepatocyte growth factor and eotaxin, as compared with recovered patients, suggesting an inability of the former subjects to maintain an immune mechanism associated with clinical recovery.16
Clinical manifestationsThe incubation period for CHIKV virus ranges between 1 and 12 days. The disease usually presents abruptly with high fever, rash, back aches and myalgia. The febrile episode lasts 3–4 days. Occasionally there is a second febrile course lasting shortly. Arthralgia and arthritis are extremely common and usually polyarticular and distal, with as many as ten joints involved. Both, small and large joints can be affected. Symmetric inflammation is common, but unilateral compromise is possible. The pain is intense and crippling, preventing patients from sleeping and ambulating properly. Articular symptoms subside within 1 to 3 weeks. Other manifestation include headache; photophobia; sore throat; abdominal pain, diarrhea and vomiting; and cervical or generalized lymphadenopathy.17
A morbilliform rash mostly non-pruritic, initially appearing in the upper limbs is the most common cutaneous manifestation. The rash may evolve into a vesiculobullous and rarely a purpuric exanthema, particularly in children. Hyperpigmentation in the centrofacial area and intertriginous ulcers can also be seen.18
Traditionally CHIKV infection was not associated with neurologic involvement, however cases of encephalitis, meningitis, acute flaccid paralysis and sensorineural hearing loss have been described in the recent outbreaks. Among neurologic symptoms, the most prevalent seem to be abnormal mental status, headache, focal deficits and seizures.19
Photophobia, retro-orbital pain and conjunctival effusion or conjunctivitis are quite common in the acute phase of the illness. Anterior uveitis is the most common serious ocular presentation and can be associated with corneal precipitates and hypopyon. Less commonly, posterior uveitis, retinitis, choroiditis and optic neuritis have been described.20
Hemorrhagic manifestations are rare; however gingivorrhagia, epistaxis, hematemesis and melena have been described in old case series.21 Abnormal bleeding, milder than the one seen in dengue fever, has a predilection for children.
A high percentage of pregnant women could be affected by CHIKV (close to 50% in a cohort in the Reunion Island). Symptoms at presentation do not differ from the general population, except for the presence of epistaxis or gingivorrhagia which were seen in 9% of pregnant females. Pregnant women may require hospitalization to rule out other conditions but the outcome of their pregnancies seem to be unaffected: the number of cesarean sections, third trimester bleeding, preterm births, still births, newborns with low weight or newborns with congenital malformations was no different when compared with uninfected women.22
Transplacental transmission is unlikely. Transmission from mother to fetus occurs exclusively at the time of delivery in the setting of intrapartum maternal viremia.23 Affected neonates develop pain, prostration, fever and thrombocytopenia within few days after birth. Some of them may have encephalopathy and intracranial bleeding with prolonged sequela.24
In the majority of patients, symptoms abate after 1–3 weeks; however some patients evolve into a chronic condition. Chronic manifestations may include monoarthritis, undifferentiated polyarthritis, joint involvement simulating rheumatoid arthritis (including fulfillment of American Academy of Rheumatology criteria, positive rheumatoid factor and characteristic erosions in the X-ray), tenosynovitis/enthesopathy, seronegative spondyloarthritis and/or back pain.25,26 The most prominent joint swelling occurs in the ankles and feet.27 The intensity of the pain is less severe, but still significant. Extra musculoskeletal manifestations such as rash, alopecia, pruritus, ocular manifestations and Raynaud's phenomenon may also occur.28 The chronic illness takes a toll on the well being of patients who score low in quality-of-life indices. They may be unable to return to work because of difficulties walking and handling objects.28–30 There is evidence that patients remain symptomatic 18–36 months and even longer after becoming infected.27,29
Patients with more prominent acute symptoms have higher risk to evolve into the chronic stage: high fevers and chills, severe malaise, notable polyarthralgia and generalized myalgia and rash may be predictors of poor outcome.25 Other risk factors for relapsing or persistent CHIKV infection include age older than 45, higher titers of CHIKV antibodies and elevated viral load.24,29 Also individuals with prior joint disease are at risk: exacerbation of psoriatic and seronegative arthritides in the setting of CHIKV infection have been described.25
It is likely that persistence of viral replication in synovial fluid is the cause of chronic manifestations as determined by detection of viral RNA and antigen in the fluid, and by persistent serum elevation of IgM antibodies.31
Although CHIKV disease has been considered self-limited, serious complications have been described as above, in addition lethality can also occur, particularly among the young, the elderly, and the immunocompromised. In Ahmedabad, India during an outbreak in 2006, all cause mortality was increased during the months of August–November, which coincided with the peak of the epidemic.32
In the other end of the spectrum, seropositive patients without clinical manifestations have also been identified.
Cases of dengue (including the hemorrhagic presentation) and CHIKV coinfection have been described (as they share the same vector) however no coinfection with yellow fever has been reported yet.
EpidemiologyCHIKV is an enzootic virus first isolated in a febrile patient during an outbreak in the Makonde Plateau in Tanzania in 1952.33 Since then cases have been reported from tropical and subtropical regions of Africa, the Indian Ocean islands and South and Southeast Asia, usually following a pattern with outbreaks occurring every 7–20 years. The largest epidemic of CHIKV occurred in 2005–2006 in the Reunion Island: about 266,000 residents of this island in the Indic Ocean (34.3% of the population) were affected. Additionally as the Island is an overseas department, many cases were exported to France.28
In 2006 CHIKV reached continental India. The infection initially affected seven states but in the last report in 2010 had extended to 18 States and territories in the Union.34 Several other countries in South East Asia have reported cases since then and CHIKV is widespread in Malaysia, Sri Lanka and Indonesia.35
In 2007 an outbreak was reported in Ravenna, Italy causing about 100 cases and at least one lethality, triggering efforts by the European Centre for Disease Prevention and Control to maintain vector control capabilities and respond effectively to the emergent outbreak.36
In December 2013 the World Health Organization reported the first local transmission of CHIKV in the Americas in the Caribbean island of Saint Martin.37 Reports of the disease ravaging other countries of South and Central America have ensued since then. Although cases in returning travelers were previously identified, the CDC described autochthonous cases in Florida in 2014. A climate based mosquito population dynamics stochastic model predicts that areas with marked season variation may become epidemic foci and potential targets for strategic vector control.38 It has been proposed that, because individuals in the US spend far less time outdoors and typically have door and window screens, and air conditioning, the extend of a potential epidemics in the United States will be less vast than in other countries.39
The first autochthonous cases of CHIKV in Brazil were confirmed in Oiapoque, Amapa State, on September 13, 2014. An epidemiological and clinical analyses of CHIKV infection occurring in Brazil between April and September of 2014, surprisingly detected in addition to the Asian lineage, several cases of the ECSA lineage in Feira de Santana, Northeast Brazil. The cases reported had not acquired the A226V mutation, but there is concern that this genetic event may occur in the future.9
Like any other vector transmitted disease the propagation of CHIKV depends on characteristics of the vector, the host and the environment.
Thanks to commercial exchange, A. albopictus has expanded to new geographic areas including more recently the Southeast of the United States and the Caribbean region.40 As mentioned previously, the A226V mutation has increased the fitness of CHIKV and its ability to replicate in A. albopictus.41
The climatic changes in Europe (and in the rest of the world) have favored the propagation of A. albopictus and in some cases the autochthonous transmission of CHIKV, such in Northern Italy in 2007 (but also cases of dengue in the south of France and in Croatia).32
Historically CHIKV infection was considered a self-limited and non-lethal disease, however 254 deaths in Reunion were attributed directly or indirectly to CHIKV, thus changing the perspective about the infection and emphasizing the role of the condition of the host in the prognosis of the disease.32
DiagnosisThe laboratory diagnosis of CHIKV depends on the quality, volume and timing of the sample obtained during the course of the disease.42 CHIKV infection can be diagnosed and confirmed by direct detection of the virus, viral RNA recognition or identification of serum specific antibodies.43
An acute phase serum sample obtained within 7 days of disease onset would likely have a high degree of viremia. This sample will be the best diagnostic option for virus isolation or nucleic acid detection.44 Both techniques are highly sensitive and specific, and results can be obtained within hours (for nucleic acid detection) or in 2 days (virus isolation).45 PCR techniques have the advantage of being faster and provide a prompt indication of the viral load in clinical samples and in the supernatant of cultures42,43,46 whereas viral isolation by means of cellular culture are slower and require a biosafety level 3 laboratory to reduce the risk of viral transmission. CHIKV isolation can be accomplished by intracerebral inoculation in mice less than a year old46 or by inoculation in mosquitoes. In vitro cellular culture methods require mosquito cells (C6/36) or other mammal cells including BHK-21, Vero and HeLa. The cytopathic effect the virus causes in these cell lines has a sensitivity comparable to in vivo methods.47
Most commonly the diagnosis of CHIKV is based on the detection of anti-CHIKV IgM o IgG in acute and convalescent samples. The diagnosis is confirmed by a four-fold rising titer between the aforementioned samples or by demonstration of specific IgM antibodies.42,43 Specific IgM antibodies are readily detected by enzyme linked immunosorbent assay (ELISA) 7 days post infection, thus making this test the preferred indicator of recent infection. Many patients may present too early in the course of the disease and the test may need to be repeated. Immunochromatography may be better for detection of IgG during the convalescent period. IgG antibodies usually persists for years whereas IgM antibodies decline to undetectable levels by 3 or 4 months, although in subjects with chronic symptoms these antibodies may persist for up to 24 months.46
TreatmentCurrently there is no specific therapy approved for CHIKV disease. The only therapeutic option is symptomatic relief. Hydration and electrolyte balance should be optimized. Paracetamol 1g three to four times a day in adults; or 50–60mg per kg body weight per day in divided doses in children; is the treatment of choice for fever, headache and/or pain according to the World Health Organization.48 Other non-steroidal analgesics are also usually recommended for pain control; however aspirin should be avoided to prevent further platelet dysfunction. In crippling cases corticosteroid use has been advocated, although there is not enough scientific information to support their use.49 French investigators who have experienced the devastating outbreak in the Reunion Island advocate use of tumor necrosis alpha inhibitors for chronic cases that fulfill the criteria for rheumatoid arthritis or seronegative spondyloarthropathy, but no supportive evidence exists beyond expert advice.
Ribavirin is a purine nucleoside analog currently used in the treatment of hepatitis C. A small study that enrolled 10 patients using ribavirin 200mg orally twice a day for 7 days showed improvement in arthralgia and returned capacity to ambulate. Unfortunately the study used non-blinded comparators and it was too small to reveal meaningful results.50 In theory combined use of ribavirin and type I interferon alpha may show a synergistic effect.
Chloroquine can inhibit viral replication in Vero cells by disrupting internalization of CHIKV containing endosomes. An open pilot study on 10 patients who completed 20 weeks of therapy showed significant improvement in the Ritchie articular index (commonly used to evaluate joint involvement in patients with rheumatoid arthritis) and in morning stiffness,51 however a small clinical trial failed to show decrease in the duration of viremia. Actually patients on chloroquine in that trial complained or worsening arthralgia as compared with placebo, but the course duration was only 5 days.52
Anterior and posterior uveitis caused by CHIKV have been treated with topical steroids and cycloplegics, and systemic steroids and acyclovir respectively. The role of acyclovir in this clinical situation is disputed.20
There are a number of drugs which may potentially have effect against CHIKV but they have not been used in clinical trials.26,53 They are summarized in Table 2. Among them siRNA, 21-23 nucleotide small interfering RNA that is homologous in sequence to E2 and ns1 genes of Chikungunya, seem to be highly promising.54
Experimental drugs for the treatment of CHIKV disease.
| Drug | Possible mechanism of action |
|---|---|
| Arbidol | Inhibits CHIKV entry |
| 6-Azauridine | Inhibits viral genome replication |
| Decanoyl-RVKR-chloromethyl ketone | Inhibits viral glycoprotein maturation |
| 5,7-Dihydroxyflavones | Inhibits viral protein translation |
| Harringtonine | Inhibits viral protein translation (specifically production of nsP3 and E2 proteins) |
| Interferon alpha | Immunomodulator |
| Mycophenolic acid | Inhibits viral genome replication |
| Peptide-conjugated phosphorodiamidate morpholino oligomer (PPMO) | Inhibits viral genome replication |
| Phenotiazine | Inhibits CHIKV entry |
| Polyinosinic acid:polycytidylic acid | Immunomodulator |
| Small interfering RNA (siRNA) | Inhibits viral genome replication |
| Trigocherrines | Inhibits viral protein translation |
Human polyvalent immunoglobulins obtained from convalescent patients with CHIKV infection have been purified and used in mouse models and exhibit a high neutralizing activity, preventing viremia.55 The use of hyperimmune serum has been successfully explored in other viral diseases, but no human trials have been attempted for CHIKV disease yet. Monoclonal antibodies have also been isolated and used as prophylactic agents in mice lacking type I IFN receptors. These antibodies have shown complete protection against lethality in the murine model.56
A case series has reported treatment for 21 patients with chronic CHIKV infection who satisfied the American College of Rheumatology criteria for rheumatoid arthritis (including a positive rheumatoid factor). All patients were treated with disease modifying antirheumatic drugs including methotrexate and TNF blockers, in addition to corticosteroids. Overall the clinical response seems to have been poor.57
PreventionThe best method to prevent CHIKV is avoidance of mosquito bites. This may be accomplished by use of repellents (preferably containing DEET), wearing of long-sleeved shirts and long pants and use of screens, bed nets and air conditioning.58
Reduction of peridomiciliary water puddles and containers may prevent mosquito proliferation.
Travelers to endemic areas need to be educated about their risks, precautions and symptom recognition.
There is potential of transmission of CHIKV via blood transfusion, although cases have not been reported yet. Sanitary authorities may need to undertake mass screening if the risk is high.59
The OX513A strain of A. aegypti is composed by radiation sterilized male insects. A study of the release of these agents in a suburb of Juazeiro, Bahia, Brazil demonstrated a reduction of 95% of the local A. aegypti population in one year. The Sterile Insect Technique (SIT) is a genetic control system that seems promising as a mean of vector control.60 Its use, among public controversy, has been imitated in Key West, the most southern region of the United States.
There is no vaccine currently available to prevent CHIKV infection. An ideal vaccine, should be of low cost, highly stable (as it will be predominantly administered in developing countries), administered in a single injection and able to stimulate rapid protection.
There are many vaccine candidates that have been tested in mouse models, one of the most intriguing ones is a non-adjuvanted, inactivated whole virus CHIKV vaccine applied by dermal application using a delivery system called Foroderm. Foroderm consists of elongated microparticles to which the whole virus is attached. Topical application in mice is followed by circulation of the particles into the lymphatic system (verified by fluorescence). A single application protected mice against viremia and disease following a virus challenge.61
In the last 2 years two vaccines have been tested in Phase 1 trials in humans: a live recombinant measles-virus-based chikungunya vaccine and a virus-like particle chikungunya virus vaccine (VRC-CHKVLP059-00-VP). Both of them were immunogenic, safe and well tolerated.62,63 More advanced phase II/III trials are hampered by the sporadic nature of the infection, which makes planning and approval of clinical trials quiet challenging.
A single vaccine has been tested in phase II trials. This was a serially passaged, plaque-purified live Chikungunya vaccine produced by the United States Army Medical Research Institute for Infectious Diseases (USAMRIID). The vaccine was injected subcutaneously and compared in a double-blinded randomized study with placebo. Ninety eight percent of recipients developed neutralizing antibodies (which persisted in 50% of cases for more than a year). The vaccine was safe and the only difference with placebo was more frequent arthalgia.64 Despite the promising results further clinical development was aborted in 2000 due to the lack of commercial interest.
Conflicts of interestThe authors declare no conflicts of interest.