Bloodstream and venous catheter-related corynebacterial infections in paediatric patients with haematological cancer were investigated from January 2003 to December 2014 at the Brazilian National Cancer Institute in Rio de Janeiro, Brazil. We observed that during cancer treatment, invasive corynebacterial infections occurred independent of certain factors, such as age and gender, underlying diseases and neutropenia. These infections were ssscaused by Corynebacterium amycolatum and other non-diphtherial corynebacteria. All cases presented a variable profile of susceptibility to antimicrobial agents, except to vancomycin. Targeted antibiotic therapy may contribute to catheters maintenance and support quality of treatment. Non-diphtherial corynebacteria must be recognized as agents associated with venous access infections. Our data highlight the need for the accurate identification of corynebacteria species, as well as antimicrobial susceptibility testing.
Oncological treatment induces severe immune suppression, rendering patients susceptible to invasive infections.1 Non-diphtherial Corynebacterium infections (NDCi) in patients with cancer have been reported with increasing frequency1–4 including medical device-associated infections. Despite the existence of international guidelines on how to perform sterile insertion and appropriate central venous catheter (CVC) maintenance and use, infection remains a common complication in these patients.5
In addition, medical experience with Corynebacterium infections in paediatric patients with cancer is currently limited. Corynebacterium striatum and Corynebacterium amycolatum were the most common isolated species in CVC-related NDCi.3 Predisposition to Corynebacterium jeikeium was demonstrated in paediatric patients with lymphoblastic leukemia.6
In this retrospective and descriptive study, we analysed the clinical, epidemiological and microbiological features of bloodstream and CVC infections caused by non-diphtherial Corynebacterium species in paediatric patients with haematological malignancies treated at the Brazilian National Cancer Institute (INCA) in Rio de Janeiro, Brazil from January 2003 to December 2014.
MethodsPatient eligibilityPatients using CVC with positive blood cultures were considered to be infected when the attending physician evaluated the clinical condition associated with fever as significant and initiated the specific antimicrobial therapy. Patients with at least two positive blood cultures for Corynebacterium species were considered to have corynebacterial bacteraemia.7 Neutropenia was defined as a neutrophil cell count lower than 1000 cells/mm.3 Patients were monitored by the Joint Commission of Infection Control and Surveillance and Nurses Committee, Outpatients Catheter and Bacteriological e-charts. This study was approved by the Research Ethics Committee at INCA, Brazil [CEP No. 139/11/ CAAE-0121.0.007.000-11] and registered in the National Commission on Ethics in Research (CONEP)].
Clinical featuresAn infectious episode was defined by the first positive blood culture for Corynebacterium (index culture). The day of collection was considered to be the onset of the infection episode. Only one episode of corynebacterial infection was recorded per patient regardless of the total number of positive blood cultures.8 Bloodstream infections (BSI) were considered as primary after laboratory confirmation and the absence of other body site infection. All primary bacteraemia events were classified as catheter-related infection (CR-BSI) if they occurred after the infection of an ostium or tunnel with a differential time for positivity of blood culture or were associated with a colony count higher than 15 CFU (Colony-forming unit) after catheter removal. Furthermore, catheter-associated infection (CA-BSI) was classified when microorganisms of another infection site did not correspond to the microorganism isolated from the blood sample obtained from the catheter.9 Secondary bacteraemia was considered when there was an infectious process at another site. Sepsis was considered when there was more than one distant site of infection.8
Microbiological analysisThe clinical isolates were analysed by the Laboratory of Microbiology at INCA. Briefly, two sets of blood samples were obtained from peripheral vein access and/or from the catheter when present and inoculated into two vials each of Bactec Plus anaerobic/aerobic. These were then incubated in a Bactec 9240 System (Cockeysville, USA). Positive blood cultures were plated into Columbia blood agar base (Detroit, USA) supplemented with 5% defibrinated sheep blood and incubated for 48 h at 37 °C. Bacterial colonies of irregular Gram-positive rods on agar plates were analysed for morphological features of corynebacterial haemolysis and pigment formation. Phenotypic profiles were determined by using API Coryne System (BioMérieux, Lyon, France). The following conventional biochemical tests and CAMP reaction were performed according to previously described methods.10 Profiles of susceptibility to antimicrobial agents (Oxoid, UK) were determined by automated microdilution tests as previously described.11 The E-test (Solna, Sweden) was also performed for vancomycin.
Statistical analysisData were converted into percentages of isolation of corynebacterial species from patients involved in the study. Data for the Chi square or Fisher exact test variables were obtained using Epi-Info version 7. Results were considered significant when p < 0.05.
ResultsDuring this study, 1.639 long-term catheters were used in paediatric patients at our Institution. A total of 25.6% of patients, all of them haematological patients, used Hickman’s catheter. Eleven cases of NDCi were identified in this group during treatment (Table 1).
Distribution analysis by gender shows that the prevalence of male patients was 63.6%. The median age of patients was 8.0 years old and they presented the following underlying haematological malignancies: Acute Lymphoblastic Leukemia (ALL) (n = 06), non-Hodgkin Lymphoma (NHL) (n = 04) and Acute Myeloid Leukaemia (AML) (n = 01). Most patients were neutropenic: three with ALL (27.3%), three with NHL (27.3%) and one with ALM (9.1%) (Table 1).
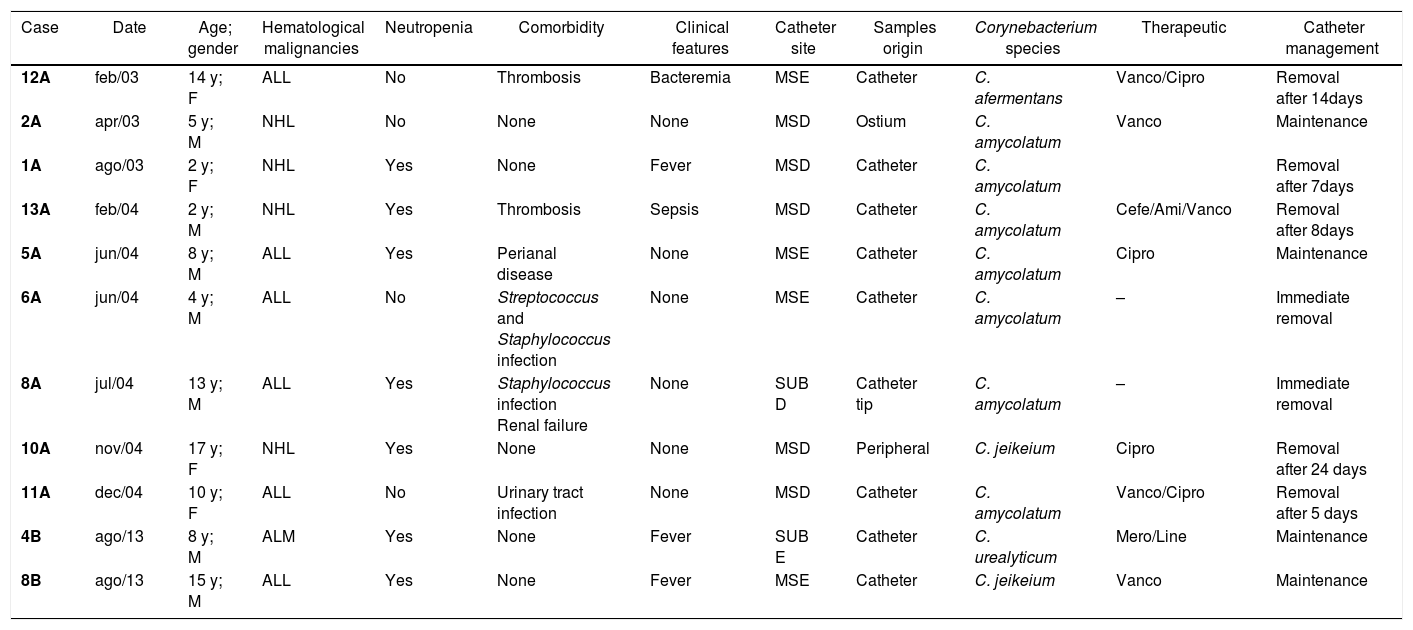
Clinical aspects of eleven haematologic paediatric cancer patients (0–17 years of age) with bacteremia caused by non-diphtherial Corynebacterium species treated at the National Cancer Institute (INCA/ RJ – Brazil) from January 2003 to December 2014.
| Case | Date | Age; gender | Hematological malignancies | Neutropenia | Comorbidity | Clinical features | Catheter site | Samples origin | Corynebacterium species | Therapeutic | Catheter management |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12A | feb/03 | 14 y; F | ALL | No | Thrombosis | Bacteremia | MSE | Catheter | C. afermentans | Vanco/Cipro | Removal after 14days |
| 2A | apr/03 | 5 y; M | NHL | No | None | None | MSD | Ostium | C. amycolatum | Vanco | Maintenance |
| 1A | ago/03 | 2 y; F | NHL | Yes | None | Fever | MSD | Catheter | C. amycolatum | Removal after 7days | |
| 13A | feb/04 | 2 y; M | NHL | Yes | Thrombosis | Sepsis | MSD | Catheter | C. amycolatum | Cefe/Ami/Vanco | Removal after 8days |
| 5A | jun/04 | 8 y; M | ALL | Yes | Perianal disease | None | MSE | Catheter | C. amycolatum | Cipro | Maintenance |
| 6A | jun/04 | 4 y; M | ALL | No | Streptococcus and Staphylococcus infection | None | MSE | Catheter | C. amycolatum | – | Immediate removal |
| 8A | jul/04 | 13 y; M | ALL | Yes | Staphylococcus infection Renal failure | None | SUB D | Catheter tip | C. amycolatum | – | Immediate removal |
| 10A | nov/04 | 17 y; F | NHL | Yes | None | None | MSD | Peripheral | C. jeikeium | Cipro | Removal after 24 days |
| 11A | dec/04 | 10 y; F | ALL | No | Urinary tract infection | None | MSD | Catheter | C. amycolatum | Vanco/Cipro | Removal after 5 days |
| 4B | ago/13 | 8 y; M | ALM | Yes | None | Fever | SUB E | Catheter | C. urealyticum | Mero/Line | Maintenance |
| 8B | ago/13 | 15 y; M | ALL | Yes | None | Fever | MSE | Catheter | C. jeikeium | Vanco | Maintenance |
y, years-old; m, months-old; NHL, non-Hodgkin lymphoma; ALL, acute lymphoblastic leukemia; ALM, acute lymphoblastic myeloma; MSD, right superior member; MSE, left superior member; SUB D, right subclavian; SUB E, left subclavian. Vanco, vancomycin; Cipro, ciprofloxacin; Cefe, cefepime; Ami, Amikacin; Mero, Meropenem; Line, Linezulid.
Data from the Fisher exact tests (95% confidence interval) revealed invasive corynebacterial infections independent of certain factors, such as age and gender (p = 0.73), underlying diseases (p = 0.82) and neutropenia (p = 0.66). We found no association between 30-day mortality and the use of LT-CVC (long-term central venous catheter) (p = 0.87).
Cases of CVC infection were mainly due to Corynebacterium amycolatum (n = 7). Two patients presented coagulase-negative Staphylococcus species and/or Streptococcus sp. isolated along with Corynebacterium amycolatum strains from clinical samples.
Other Corynebacterium species were isolated as well: C. jeikeium (n = 2), C. afermentans (n = 1), C. urealyticum (n = 1). Cases of bacteraemia due to C. jeikeium were observed in two neutropenic patients (Table 1).
C. afermentans infection was diagnosed in a non-neutropenic female teenager. This patient presented a septic thrombosis despite endovenous therapy with vancomycin and ciprofloxacin and the catheter was removed. C. urealyticum was isolated from a non-neutropenic child and the catheter was preserved after venous treatment with amikacin and vancomycin.
Most patients had a good clinical response after catheter removal (n = 7) or antimicrobial treatment protocol preserving venous access (n = 4). Infections may increase the incidence of catheter removal in patients with cancer (p = 0.04) (Table 1).
C. amycolatum strains demonstrated variable sensitivity to the antimicrobial agents tested and C. jeikeium strains presented a MDR (multidrug resistant) profile. Vancomycin induced a susceptibility of 0.28 mcg/mL (0.13–0.5) in microbiological testing. Vancomycin and linezolid were the only antimicrobial agents with a broad activity against Corynebacterium isolates with 100% susceptibility.
Since January 2009, due to the implementation of a new treatment strategy for catheter bloodstream-related infections (CBSRI protocol), catheters were kept in place in most of cases.
DiscussionRecent studies support a new perspective of venous access in oncology. Improvements in all these devices bring better quality of life and benefits to patients.
The use of CVCs may increase the risk for corynebacterial bloodstream infection in paediatric oncology patients.1,3 Researchers from St. Jude Children's Hospital, USA, reported 17 cases of infections caused by coryneform bacteria in paediatric patients with cancer (with a median age of 11.2 years old). The most common species isolated were Corynebacterium striatum, C. amycolatum and Microbacterium species. Corynebacteria species were isolated from 5.9% febrile neutropenic children with neoplastic disease.3,12 In other studies, C. amycolatum was also the predominant species isolated from samples of cancer patients who had hospital infections in Asia.13 Some reports suggest the predisposition of C. amycolatum to adhering to catheters inserted into patients with cancer.14,15 It has been shown that paediatric oncologic patients have a predisposition to C. jeikeium infection.6 In previous studies, C. jeikeium was mainly isolated from neutropenic patients with haematological disorders.4,6. In the present study, C. jeikeium was isolated from only two neutropenic patients. Similar to other studies, both patients were treated with vancomycin, but the catheter was not removed in only one case.
Invasive C. urealyticum infections are unusual and this species is mostly associated with urinary tract infections.16. Our study was the first to report the isolation of C. urealyticum from a venous catheter in children with a non-urinary infection.
Corynebacterium striatum are recognized with true infectious agents when isolated in cultures. The biggest problem is in correctly identifying and evaluating sensitivity. Review of C. striatum studies demonstrate susceptibility to vancomycin and linezolid most often.17
The rates of antibiotic resistance at the hospital demonstrate there were changes in the incidence, treatment, and evolution of corynebacteria bacteraemia after a stricter antibiotic control (CBSRI protocol).18 Our results corroborate that vancomycin remains the best option for empiric treatment of catheter-related Corynebacterium infection.19,20
The limitations of this study include its retrospective design and a single institution data.
In conclusion, CVC infections in paediatric oncology patients may be caused by Corynebacterium amycolatum and other non-diphtherial corynebacteria. The implementation of new strategies to control catheter infection and the routine practice of the CBRSI protocol are difficult but extremely important. The data collected in our study also highlight the need for an accurate identification of corynebacteria species, as well as antimicrobial susceptibility testing. Vancomycin is still considered the first choice to control infections caused by most Gram-positive organisms.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported from the National Post-Doctoral Program – PNPD (CAPES/MEC).