Little is known about factors associated with carbapenem-resistant Klebsiella pneumoniae infections in pediatric patients, who are initally colonized with carbapenem-resistant Klebsiella pneumoniae.
Materials and methodsA retrospective case–control study was conducted involving pediatric and neonatal intensive care units throughout a five-year period (January 2010–December 2014). Clinical and microbiological data were extracted from Hospital Infection Control Committee reports and patients’ medical records. Risk factors were assessed in carbapenem-resistant Klebsiella pneumoniae colonized patients who developed subsequent systemic infection (cases) and compared to carbapenem-resistant Klebsiella pneumoniae colonized patients who did not develop infection (controls).
ResultsThroughout the study period, 2.6% of patients admitted to neonatal intensive care units and 3.6% of patients admitted to pediatric intensive care units had become colonized with carbapenem-resistant Klebsiella pneumoniae. After a mean of 10.6±1.9 days (median: 7 days, range: 2–38 days) following detection of colonization, 39.0% of the carbapenem-resistant Klebsiella pneumoniae colonized patients in pediatric intensive care units and 18.1% of carbapenem-resistant Klebsiella pneumoniae colonized patients in neonatal intensive care units developed systemic carbapenem-resistant Klebsiella pneumoniae infection. Types of systemic carbapenem-resistant Klebsiella pneumoniae infections included bacteremia (n=15, 62.5%), ventilator-associated pneumonia (n=4, 16.6%), ventriculitis (n=2, 8.3%), intraabdominal infections (n=2, 8.3%), and urinary tract infection (n=1, 4.1%). A logistic regression model including parameters found significant in univariate analysis of carbapenem resistant Klebsiella pneumoniae colonization and carbapenem resistant Klebsiella pneumoniae infection groups revealed underlying metabolic disease (OR: 10.1; 95% CI: 2.7–37.2), previous carbapenem use (OR: 10.1; 95% CI: 2.2–40.1), neutropenia (OR: 13.8; 95% CI: 3.1–61.0) and previous surgical procedure (OR: 7.4; 95% CI: 1.9–28.5) as independent risk factors for carbapenem-resistant Klebsiella pneumoniae infection in patients colonized with carbapenem-resistant Klebsiella pneumoniae. Out of 24 patients with carbapenem resistant Klebsiella pneumoniae infection, 4 (16.6%) died of carbapenem-resistant Klebsiella pneumoniae sepsis.
ConclusionAsymptomatic colonization with carbapenem-resistant Klebsiella pneumoniae in intensive care units of pediatric departments should alert health care providers about forthcoming carbapenem-resistant Klebsiella pneumoniae infection. Those carbapenem-resistant Klebsiella pneumoniae colonized patients at risk of developing infection due to carbapenem-resistant Klebsiella pneumoniae may be targeted for interventions to reduce subsequent infection occurence and also for timely initiation of empirical carbapenem-resistant Klebsiella pneumoniae active treatment, when necessary.
Klebsiella pneumonia is a Gram negative rod belonging to Enterobacteriaceae family. Carbapenem resistance in Klebsiella pneumonia is mainly associated with K. pneumoniae carbapenemase.1–3 It is a carbapenem hydrolizing beta-lactamase encoded by transmissible plasmids, which facilitate spread of the enzyme among bacterial species.4 During the last decade, infections due to carbapenem resistant Klebsiella pneumoniae (CRKP) has been reported throughout the world, spreading from the United States in 2001.2 Outbreaks of CRKP has been reported in several countries.5,6 Rapid and global dissemination of CRKP is of great concern in health care facilities. It can cause diverse infections including primary bacteremia, urinary tract infections, pneumonia, intra-abdominal infections, and wound infections. Crude mortality rate of CRKP infections ranges from 30% to 44%.7–9 It increases strikingly to 71.9%, when in the case of bacteremia.10 CRKP strains have higher mortality rate compared to strains susceptible to carbapenem.7,10
Prompt initiation of appropriate antibiotic therapy for CRKP infections is crucial for patient survival.8,11,12 However, appropriate antibiotics like colistin are not administered routinely to these patients until the cultures yield CRKP isolate. Knowing that a patient is colonized by CRKP may be beneficial in deciding to start empirical CRKP active treatment in suspicion of a Gram negative infection.
Risk factors for acquisition of CRKP colonization have been mainly assessed in studies involving adult patients and were identified as antibiotic exposure, especially carabapenem, intensive care unit stay, prolonged hospitalization, poor functional status, invasive devices.7,8,13–15 CRKP colonization of the host is a major predisposing factor for developing subsequent CRKP infection.7,16 The studies which evaluate the frequency and risk factors of CRKP infection occurrence in patients rectally colonized with CRKP are limited in the pediatric population. Therefore, the present study was planned to determine the frequency distribution and description of CRKP infections in rectally colonized patients, who were admitted in pediatric and neonatal intensive care units over five years, as well as to identify associated risk factors for developing subsequent CRKP infection.
Materials and methodsHospital setting, data collection and study designThis report is a retrospective case–control study conducted in a tertiary university teaching hospital in Istanbul, Turkey. Since January 2010, infection control nurses assigned from the Hospital Infection Control Committee (HICC) have performed active surveillance of CRKP and vancomycin-resistant enterococci (VRE) rectal colonization in selective high risk wards, including pediatric and neonatal intensive care units (PICU and NICU, respectively). Rectal swabs of patients admitted to PICU or NICU were routinely screened once a week for the presence of CRKP and VRE. An infection control nurse prospectively tracked all health care associated infections occurring at the PICU and NICU, together with the results of rectal surveillance and reported these data monthly to the HICC. This database was reviewed to identify pediatric patients who were rectally colonized with CRKP and those who developed subsequent CRKP infection from January 2010 to December 2014.
Medical, microbiological and laboratory data were extracted from HICC reports and the patients’ own medical records. The data collected from patients with CRKP colonization included demographics, hospital unit at the time of CRKP detection, underlying diseases, invasive procedures within previous four weeks, duration and types of antibiotics use, absolute neutrophil and lymphocyte count at the time of colonization, and type of CRKP infection. In order to identify risk factors for CRKP infection among colonized patients, a case–control study was performed comparing the characteristics of CRKP colonized patients who developed subsequent CRKP infection (cases) with CRKP colonized patients who did not developed CRKP infection (controls). Variables related to the outcome of the infected patients like treatment of CRKP infection, treatment outcome, length of hospital stay, and crude in hospital mortality rate were also recorded.
The study protocol was approved by the Institutional Review Board.
DefinitionsCRKP colonization (CRKP-C) was defined as positive rectal swab in a patient with no clinical specimens yielding CRKP. CRKP infection (CRKP-I) was defined as a positive clinical specimen together with signs and symptoms of infection in a patient previously colonized with CRKP.
Patients with known CRKP colonization prior to admission or found to be positive in the first 72h of admission were defined as imported cases. On the other hand, the term cross-transmission was used for the patients who had negative rectal swabs in first 72h of admission and who acquired CRKP colonization subsequently during the current hospitalization. Undetermined cases were those who could not be sampled in the first 72h of admission.
HICC determined whether CRKP represented a case of infection or colonization based on the definitions of the Centers for Disease Control and Prevention.17
Microbiological procedureSurveillance cultures were collected on sterile stuart transport medium (Pipe star Kft, Budapest, Hungary). After transportation to the microbiology laboratory, rectal swabs were inoculated on chromogenic agar supplemented with 1mg/L meropenem (HiCrome, Himedia, India). After 18–24h inoculation at 37°C, dark-blue colonies were identified at species level by conventional biochemical methods. Antibiotic susuceptibility testing was perfomed by utilizing agar disk diffusion method (Becton, Dickenson & Company, USA) in accordance with the Clinical and Laboratory Standards Institute breakpoints.18 Carbapenem resistance was confirmed by detection of MIC values for carbapenems (imipenem, meropenem, ertapenem) via E-test method (bioMérieux, France).
StatisticsAll statistical analysis was performed with the statistical package for social science (SPSS) for Windows version 21.0. Normality was assessed by Shapiro–Wilk test and histogram graphics. Data are presented as median, minimum, maximum, frequency, and percentage. Categorical variables between groups were compared with the χ2 test or Fisher exact test when the expected cell size was below 5. Normally distributed continuous variables were compared by Student's t test. Mann–Whitney U test was used for continuous variables, which were not normally distributed. All p-values were based on two tailed statistical analyses and p-values less than 0.05 were considered statistically significant. Risk factors for CRKP infection identified in the univariate analysis (p≤0.1) were included in the multivariate analysis. Logistic regression analysis was performed to determine risk factors independently associated with subsequent development of CRKP infection.
ResultsBetween January 2010 and December 2014, 2805 patients were admitted to the intensive care units of our institution (PICU: 1134 patients, NICU: 1671 patients). Throughout the study period, a total of 85 patients (3.03%) were rectally colonized with CRKP. Out of those 85 patients carrying CRKP, 44 patients were admitted at the NICU (2.6% of all patients admitted to NICU) and 41 patients were admitted at the PICU (3.6% of all patients admitted to PICU). No significant difference was found between PICU and NICU regarding CRKP colonization rate (p>0.05).
Patients’ ages ranged from 1 to 205 months (median: 3 months; mean: 30.7±5.7 months) and 54.1% were male. On the basis of criteria described in Section “Materials and methods”, the majority of patients acquired CRKP by cross-transmission (n=75, 88.2%), and 9 patients (10.5%) had imported CRKP. There was only one undetermined case.
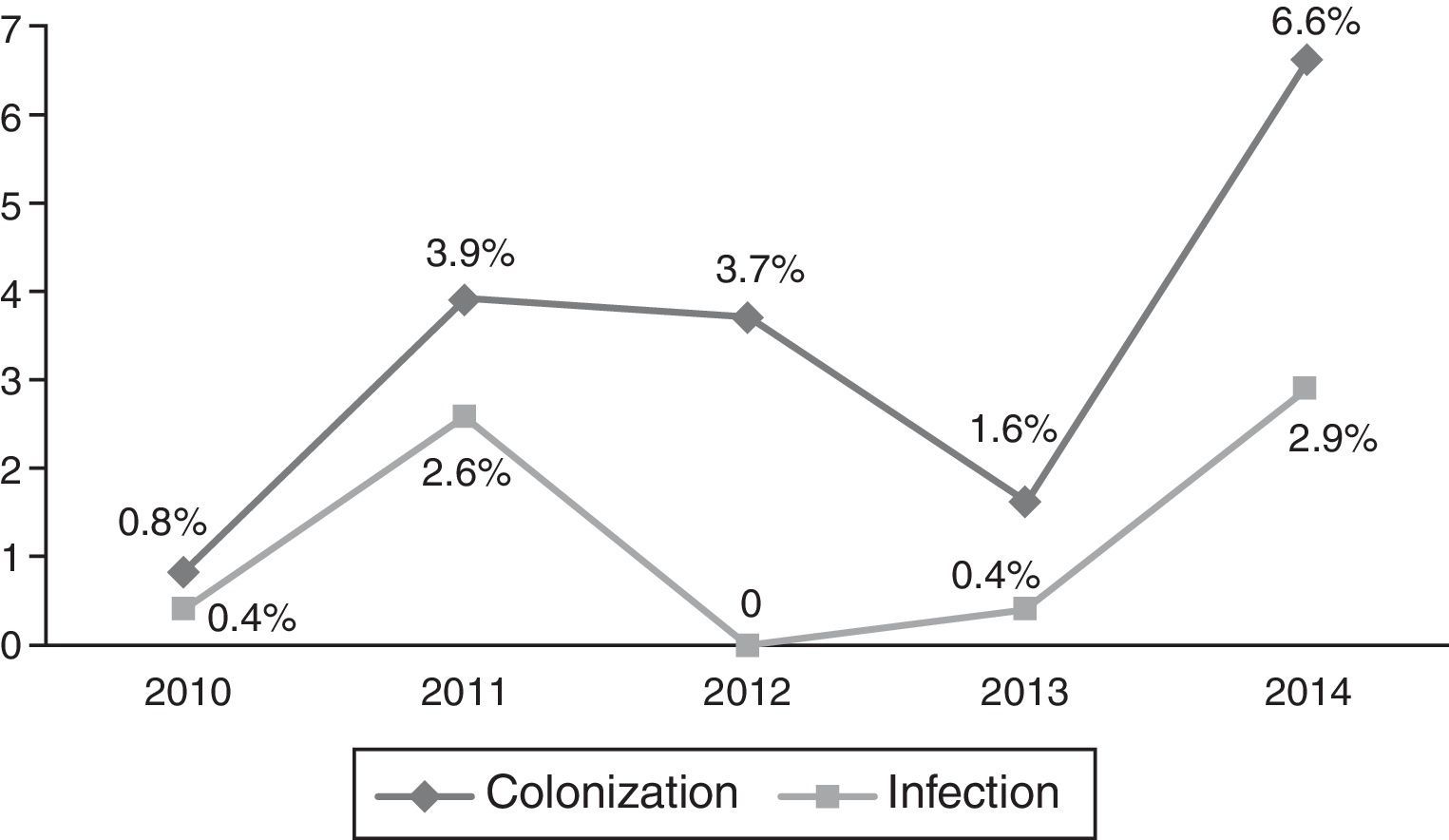
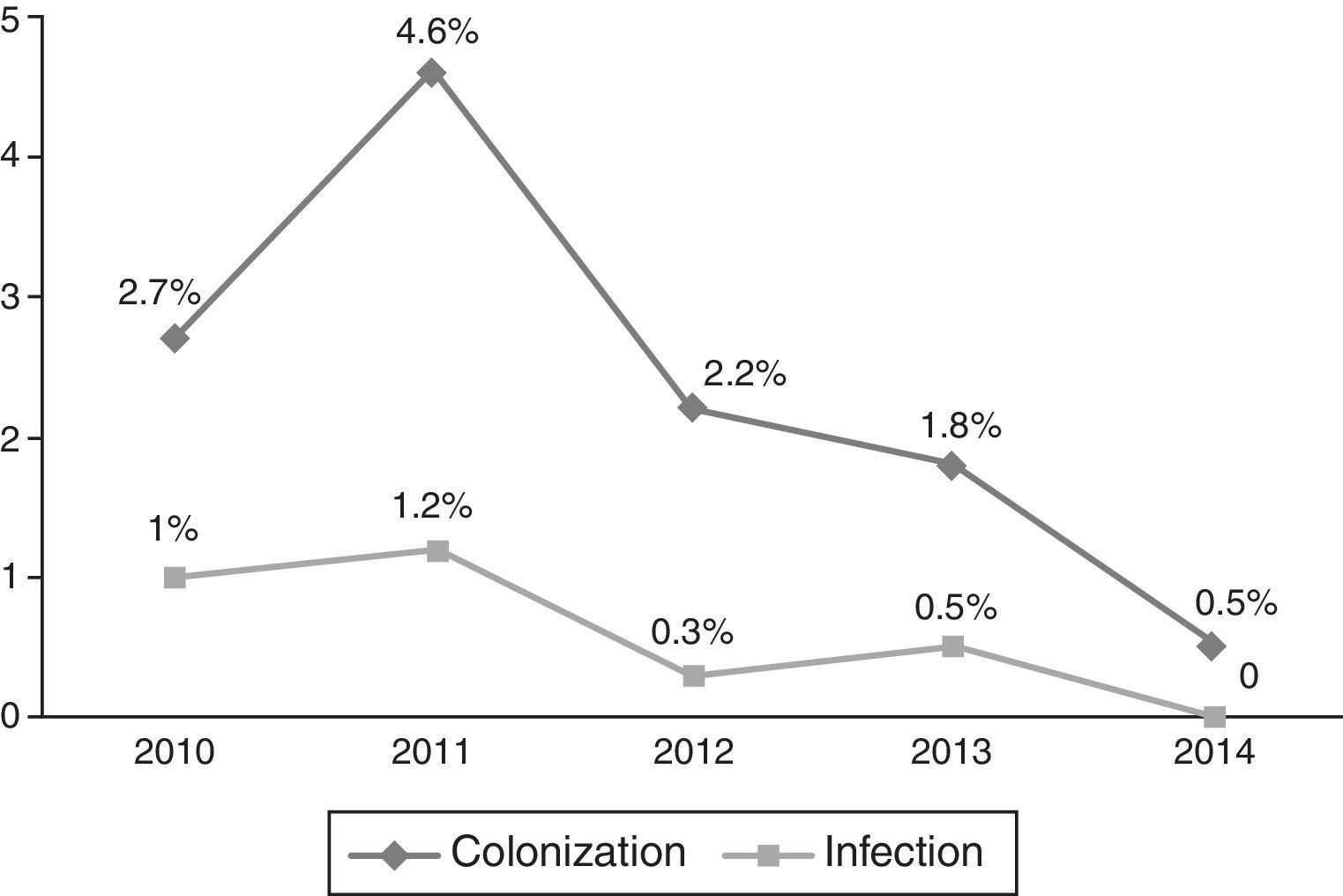
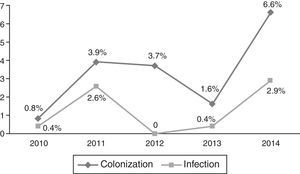
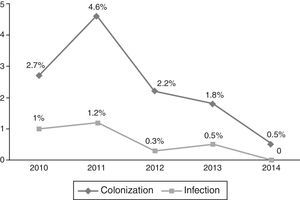
Occurrence of CRKP infectionFor all patients, frequency of subsequent CRKP infection among CRKP carriers was 28.2% (24 out of 85). In the NICU, 8 patients out of 44 (18.1%) developed subsequent CRKP infection, compared 16 patients out of 41 (39%) in the PICU (p=0.03). On average, patients developed CRKP infection 35.50±28.78 days (median: 33 days, range: 6–143 days) after admission and 10.6±1.9 days (median: 7 days, range: 2–38 days) after detection of CRKP colonization. The yearly distribution of CRKP-C and CRKP-I cases among all admitted patients to PICU and NICU in that particular year is presented in Figs. 1 and 2, respectively.
Types of CRKP infection included primary bacteremia in 15 patients (62.5%), ventilator-associated pneumonia in 4 patients (16.6%), ventriculitis in 2 patients (8.3%), intraabdominal infections in 2 patients (8.3%), and urinary tract infection in 1 patient (4.1%). Two of the primary bacteremia were catheter associated. Ventriculitis developed in 2 premature newborns with extraventricular drainage catheter inserted for hydrocephalus.
Risk factors for subsequent CRKP infectionPatients with CRKP-I were older in age than those with CRKP-C (53±14.7 and 23.5±5.8 months, p=0.039). Length of hospital stay before detection of CRKP colonization was not different in CRKP-C and CRKP-I groups (30.6±4.4 and 24.5±5.2 days, respectively; p=0.79).
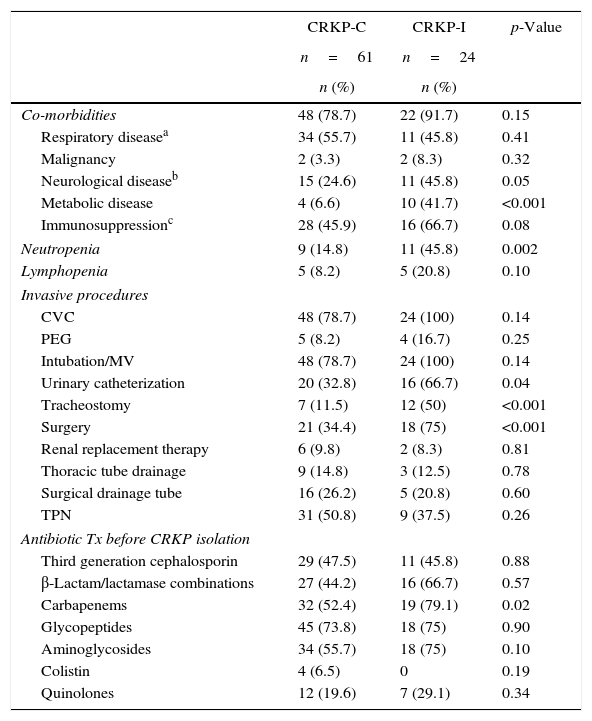
Table 1 summarizes univariate analyses of possible risk factors associated with subsequent CRKP infection among patients colonized with CRKP. Presence of underlying chronic disease was similar in CRKP-C and CRKP-I groups. However, when individual comorbid diseases were evaluated, CRKP infected patients had suffered more frequently from metabolic and neurological diseases (p=<0.001 and 0.05, respectively). When neutrophil counts were compared at the time of CRKP colonization detection, CRKP colonized patients who developed subsequent CRKP infection were more likely to have neutropenia than those patients who did not developed subsequent CRKP infection (p=0.02). Individual invasive procedures during their ICU stay differred between the groups. Patients with CRKP-I had more urinary catheterization, tracheostomy, and previous surgical procedure than patients with CRKP-C (p=0.04, p<0.001, p<0.001, respectively). Total duration of antibiotic use in patients of CRKP-C and CRKP-I groups were similar (16.1±1.2 and 10.2±2.0 days, respectively; p=0.21). Among different antibiotic groups, carbapenems were more frequently used in patients with CRKP-I than patients with CRKP-C (p=0.02).
Clinical characteristics of patients with CRKP-C and CRKP-I.
| CRKP-C | CRKP-I | p-Value | |
|---|---|---|---|
| n=61 | n=24 | ||
| n (%) | n (%) | ||
| Co-morbidities | 48 (78.7) | 22 (91.7) | 0.15 |
| Respiratory diseasea | 34 (55.7) | 11 (45.8) | 0.41 |
| Malignancy | 2 (3.3) | 2 (8.3) | 0.32 |
| Neurological diseaseb | 15 (24.6) | 11 (45.8) | 0.05 |
| Metabolic disease | 4 (6.6) | 10 (41.7) | <0.001 |
| Immunosuppressionc | 28 (45.9) | 16 (66.7) | 0.08 |
| Neutropenia | 9 (14.8) | 11 (45.8) | 0.002 |
| Lymphopenia | 5 (8.2) | 5 (20.8) | 0.10 |
| Invasive procedures | |||
| CVC | 48 (78.7) | 24 (100) | 0.14 |
| PEG | 5 (8.2) | 4 (16.7) | 0.25 |
| Intubation/MV | 48 (78.7) | 24 (100) | 0.14 |
| Urinary catheterization | 20 (32.8) | 16 (66.7) | 0.04 |
| Tracheostomy | 7 (11.5) | 12 (50) | <0.001 |
| Surgery | 21 (34.4) | 18 (75) | <0.001 |
| Renal replacement therapy | 6 (9.8) | 2 (8.3) | 0.81 |
| Thoracic tube drainage | 9 (14.8) | 3 (12.5) | 0.78 |
| Surgical drainage tube | 16 (26.2) | 5 (20.8) | 0.60 |
| TPN | 31 (50.8) | 9 (37.5) | 0.26 |
| Antibiotic Tx before CRKP isolation | |||
| Third generation cephalosporin | 29 (47.5) | 11 (45.8) | 0.88 |
| β-Lactam/lactamase combinations | 27 (44.2) | 16 (66.7) | 0.57 |
| Carbapenems | 32 (52.4) | 19 (79.1) | 0.02 |
| Glycopeptides | 45 (73.8) | 18 (75) | 0.90 |
| Aminoglycosides | 34 (55.7) | 18 (75) | 0.10 |
| Colistin | 4 (6.5) | 0 | 0.19 |
| Quinolones | 12 (19.6) | 7 (29.1) | 0.34 |
CRKP, carbapenem-resistant Klebsiella pneumoniae; CRKP-C, CRKP colonized patients who did not developed subsequent CRKP infection; CRKP-I, CRKP colonized patients who developed subsequent CRKP infection; CVC, central venous catheter, PEG, percutaneous gastroenterostomy; MV, mechanical ventilation; TPN, total parenteral nutrition; Tx, treatment.
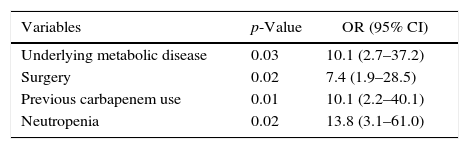
A logistic regression analysis including parameters with a p-value of ≤0.1 in univariate analysis revealed underlying metabolic disease, previous carbapenem use, neutropenia, and previous surgical procedure as independent risk factors for subsequent CRKP infection in patients colonized with CRKP (Table 2).
Multivariate analysis of risk factors for CRKP infection in patients colonized with CRKP.
| Variables | p-Value | OR (95% CI) |
|---|---|---|
| Underlying metabolic disease | 0.03 | 10.1 (2.7–37.2) |
| Surgery | 0.02 | 7.4 (1.9–28.5) |
| Previous carbapenem use | 0.01 | 10.1 (2.2–40.1) |
| Neutropenia | 0.02 | 13.8 (3.1–61.0) |
CRKP, carbapenem resistant Klebsiella pneumonia.
Total length of hospital stay was longer in patients with CRKP-I than those with CRKP-C (70.3±8.4 and 48.6±5.5 days, respectively; p=0.01). Crude in hospital mortality rate was higher in CRKP-I group in the limit of statistical significance (n=4 (6.5%) in CRKP-C vs n=7 (29.1%) in CRKP-I, p=0.05). Out of 24 patients with CRKP-I, 4 (16.6%) died of CRKP sepsis.
DiscussionIn daily practice, we are experiencing increasingly rates of CRKP infection. Treatment of these infections is more challenging for pediatricians due to limited appropriate antibiotic use groups in pediatrics. This real life problem in addition to the scarcity of pediatric studies on this issue prompted us to investigate the risk of subsequent CRKP infection of our CRKP colonized patients admitted to pediatric and neonatal intensive care units.
Overall, the rate of subsequent a nosocomial CRKP infection in colonized patients was 28.2%, and PICU had significantly higher infection rate than NICU (39% and 18.1%, respectively). This discrepancy can be explained by the higher frequency of more complicated patients with severe underlying diseases at the PICU compared to NICU, which mainly cares for premature neonates in their way to thrive. To date, there is no published study that has investigated the prevalance of neonatal colonization and subsequent infection with CRKP. In a pediatric study from Turkey, evaluating all pediatric units for a period of 7 months, a much higher colonization rate (29.5%), but a lower infection rate (3.4%) in colonized patients was found.19 In that Low infection rate in that study reflects the fact that it did not include only high risk patients, in contrary to our studied sample. Adult studies have reported a wide range of CRKP infection rates among colonizers (8.8–42%).20,21 These differences observed in colonization and subsequent infection rates among studies may be linked to inclusion of different patient populations, variations in infrastructural properties, and implementation of infection control programs. High colonization rates may indicate poor infection control measures inadequate to prevent horizontal transmisson.16 On the other hand, high infection rates may be associated with patient complexity requiring more invasive procedures and longer ICU stay under high colonization pressure.
Yearly distribution of colonization/infection rates gives the impression of a gradually increasing trend, especially at the PICU. In 2013–2014, a substantial increase both in colonization and infection was observed at the PICU. Because these cases were not clustered in time, but dispersed throughout the year, it was not considered as an outbreak. No change has occurred in physical conditions and staff structure in that year. It might have been a reflection of a considerably increasing frequency of carbapenem resistance in Klebsiella isolates in our country also, which has been shown recently in an adult study.22
Literature review reveals primarily adult studies, which defined several risk factors for development of CRKP infection in colonized patients.7,8,13–15,20 Serious underlying diseases like diabetes mellitus, malignancies, or renal disease have been previously reported risk factors for emergence of infections due to resistant microorganisms.9,13–16,20 In this study, underlying neurological and metabolic diseases were more frequently seen in CRKP-I group and the former was found to be an independent risk factor for subsequentCRKP infection. Metabolic diseases, which are predominatly diseases of the childhood, was first reported to be an independent risk factor for CRKP infection, which may be due to the scarcity of pediatric studies on this issue. Neutropenia was determined as another risk factor for CRKP infection in colonized patients. It is a rarely studied risk factor in similarly designed studies.
In the current study, urinary catheterizaton, tracheostomy, and previous surgical procedure were found to be more frequently performed in patients with CRKP-I. On multivariate analysis, previous surgical procedure was found as an independent risk factor. Surgery is a well known risk factor for infection with resistant microrganisms like CRKP, as shown in other published studies as well.15,21 Both colonization and infection have been linked to the use of invasive procedures.9,14,16,20 They facilitate the entry of the microrganism to the host. However, for this entry to occur, the microrganism should already exist in the natural or man-made environment. So, because invasive procedures are not avoidable in ICU patients most of the time, implementation and good compliance of infection control measures to prevent colonization of the environment and the patient are always key points for reducing nosocomial infections with resistant microorganisms.
Although it is generally accepted that there is a link between antibiotic usage and emergence of resistant microorganisms, literature review on this issue reveals conflicting results. As there are studies reporting antibiotic exposure as an independent risk factor for CRKP infection,7,14,19,20,23 there are also some other studies revealing no correlation between antimicrobials and isolation of CRKP.15,16,21,24 The current work found no difference in total duration of antibiotic usage between the CRKP-C and CRKP-I groups, because virtually all patients admitted to an ICU were heavily treated with antibiotics before CRKP detection. However, when individual antibotic groups were evaluated, carbapenem exposure was found to be a risk factor for subsequent CRKP-I. This finding is in accordance with other studies that have reported strong association of exposure to carbapenem agents and CRKP infection.7,14,19,25 Carbapenem use may cause selective pressure on persistence of the resistant microrganism, thereby increasing infection risk. Although there are studies indicating no effect of change in antimicrobial use in reducing CRKP infections,26 we think thata the use of carbapenem agents should be minimized, together with implementation of appropriate and strict infection control programs to combat emergence and spread of CRKP strains.
This study has several limitations. First, we could not determine types of carbapenameses harbored by CRKP strains, because this was a retrospective study and molecular analysis were not routinely performed in our institution. Second, being a study with a retrospective design, it lacks enough strength to establish a casual relationship between possible risk factors for CRKP infection. Besides, there may be additional unmeasured variables associated with CRKP infection. Third, we could not implement a comorbidity index, which would measure the severity of comorbid conditions and minimize any selection bias, due to difficulties resulting from the retrospective nature of the study design. Fourth, we did not have data about compliance of health care workers to infection control measures. It is obviously an impotant risk factor for acquisiton of CRKP, but its role in infection occurrence in patients who are already colonized, which is the focus of our study, remains to be answered in prospectively designed studies. Data are limited regarding CRKP infection development in critically ill pediatric and neonatal patients colonized with CRKP. Nonetheless, despite its limitations, this study provides important information regarding infection control in this population.
In conclusion, our data highlights the importance of CRKP colonization, which resulted in infection in nearly half of the patients admitted to the PICU and one fifth of the patients in the NICU. We demonstrated several independent risk factors for developing CRKP infection in patients initially colonized with CRKP, including underlying metabolic disease, neutropenia, previous surgical procedure, and previous carbapenem use. Patients with CRKP colonization especially those having these clinical factors should be targeted for interventions to reduce the risk of subsequent clinical infection. They should also be carefully followed up regarding signs and symptoms of infection in order to timely initiation of empirical active treatment against CRKP.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Hayriye Vehid, PhD, Department of Family Health, Institute of Pediatrics, Istanbul University, Istanbul, Turkey for her support in the statistical evaluation of this report.
The study was carried out in Istanbul University, Istanbul Medical Faculty, Department of Pediatric Infectious Diseases, Millet Street, Çapa 34093, Fatih, Istanbul, Turkey.