Vaccines in development against Group B Streptococcus (GBS) should contain the most prevalent capsular genotypes screened in the target population. In low- and middle-income countries epidemiological data on GBS carriage among pregnant women, a prerequisite condition for GBS neonatal sepsis, is needed to inform vaccine strategies.
ObjectiveTo investigate the prevalence of different GBS capsular genotypes that colonizes at-risk pregnant women in a private maternity hospital in São Paulo, Brazil.
MethodsGBS strains isolated in routine maternity procedures from at-risk pregnant women from 2014 to 2018 were confirmed by mass spectrometry (MALDI-TOF) with subsequent DNA extraction for identification of capsular genotype through polymerase chain reaction (PCR). Demographic and gestational data were analyzed.
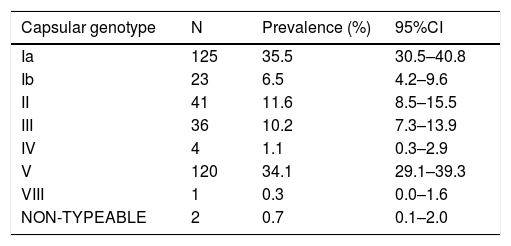
ResultsA total of 820 Todd-Hewitt broths positive for GBS were selected for streptococcal growth. Recovery and confirmation of GBS by MALDI-TOF were possible in 352. Strains were processed for determination of capsular genotype by PCR. From the total of 352 GBS isolates, 125 strains (35.5%) were genotyped as Ia; 23 (6.5%) as Ib; 41 (11.6%) as II; 36 (10.2%) as III; 4 (1.1%) as IV; 120 (34.1%) as V and 1 strain (0.3%) as VIII. Two isolates (0.7%) were not genotyped by used methodology. No statistically significant correlation between gestational risk factors, demographic data and distribution of capsular genotypes were found.
ConclusionsGBS capsular genotypes Ia, Ib, II, III, and V were the most prevalent isolates colonizing at risk pregnant women in the present study. The inclusion of capsular genotypes Ia and V in the composition of future vaccines would cover 69.6% of capsular genotypes in the studied population. No statistically significant differences were observed between capsular genotype and gestational and demographic data and risk factors.
Group B Streptococcus (GBS) is an important etiologic agent related to neonatal sepsis and severe infections in pregnant women, elderly, and individuals with chronic and immunocompromising diseases.1–3 Maternal genitourinary and gastrointestinal tract colonization are considered the primary risk factor for neonatal disease. The infection can occur through vertical or ascendant via, during delivery or after membrane rupture.4,5 A recent systematic review reported an overall incidence of invasive GBS disease among young infants of 0.53 per 1000 live births, with the highest incidence in Africa (1.12) and lowest in Asia (0.30), with a mean case fatality rate of 8.4%.6
It is estimated that 10–30% of pregnant women present their genitourinary or gastrointestinal tracts colonized by GBS and that half of their newborns will be infected. When an adequate prophylaxis is not performed, about 1 to 2% of these infants will develop neonatal invasive disease.7 However, an important difference regarding frequencies of maternal colonization may be observed according to region, ethnical and socioeconomic characteristics. In Brazil, previous data on prevalence of GBS carriage are highly diverse, although most recent studies found rates as high as 28.4%.8,9
In addition to the GBS colonization prevalence, an important aspect to be analyzed is the distribution of capsular genotypes, since some are associated with more virulent clones and a consequent greater potential to cause invasive disease.10 Ten different GBS capsular genotypes are identified and classified according to the composition of its capsular polysaccharide: Ia, Ib, II, III, IV, V, VI, VII, VIII and IX.11,12 However, a 2017 meta-analysis found that five of them (Ia, Ib, II, III, and V) are responsible for 97% of invasive isolates worldwide.6
Currently, women with GBS colonization during pregnancy and those presenting risk factors to develop or to transmit GBS are recommended to receive intravenous intrapartum antibiotic prophylaxis to prevent early-onset GBS disease. The use of intrapartum antibiotic prophylaxis, indicated to at-risk or GBS colonized pregnant women, proved to be highly effective to prevent early-onset disease among infants born to women with GBS colonization. However, this strategy does not impact the late-onset disease incidence, when most cases of meningitis occurs, and is not widely implemented in several low and middle-income countries.5,13,14
In this scenario, active immunization of women during pregnancy aiming to transfer genotype-specific anti-capsular antibodies to the fetus (passive immunization) represents a promising strategy to reduce the burden of GBS disease among infants. Therefore, the knowledge on the prevalence of different capsular genotypes is essential to guide capsular polysaccharide-based candidate vaccines composition.15,16
In Brazil, few data are available on the distribution of different GBS capsular genotypes isolated in pregnant women. GBS prevalence varies widely in national literature, ranging from 4.2% to 28.4%, considering studies published from 2008 to 2018 in Northeast, Southwest and South geographic regions. Despite the lack of consensus, capsular genotype Ia is the most frequent in some studies.17 Thus, this study was conducted aiming to investigate the prevalence of different GBS capsular genotypes that colonize at-risk pregnant women in a private maternity hospital of São Paulo, Brazil. As secondary objectives, the study aimed to assess the relationship between GBS capsular genotypes and characteristics of women, pregnancy and delivery, and to identify capsular genotypes potentially candidates to be included in future group B Streptococcal vaccines.
Materials and methodsStudy designA cross-sectional study was developed in a complex of private maternity hospitals in São Paulo city, Brazil (Hospital e Maternidade Santa Joana and ProMatre Paulista), and in the Special Laboratory of Clinical Microbiology (Laboratório Especial de Microbiologia Clínica - LEMC) of São Paulo Federal University. The study was approved by the research ethics committee of São Paulo Federal University (approval number 933.138). To ensure patients privacy, all data were anonymized before study's assessments. An informed consent was waived by the ethics committee.
Study population and sample collectionParticipating maternity hospitals routinely collect vaginal and anal swabs, in a single tube and one sowing, at hospital admission from at-risk pregnant women in order to investigate GBS colonization and to perform intrapartum antibiotic prophylaxis. A convenience sample was considered including all at-risk pregnant women attended on selected hospitals in 2014–2018 period. At-risk pregnant women were considered as those with membranes rupture for more than 18 h, gestational age less than 37 weeks, presenting fever or chorioamnionitis signs.18
Phenotypical sample processingAt maternity hospitals, collected swab samples were placed into Stuart medium and inoculated in Todd-Hewitt broth, supplemented with gentamicin and nalidixic acid (Probac do Brasil®, Brazil). After incubation for 18–24 h at 35 °C, one drop of the broth was inoculated on a plate of Todd-Hewit Blood Agar (Probac do Brasil®, Brazil) with hemolysin strip perpendicularly, and re-incubated for 24 h with subsequent reading of the camp test. Latex agglutination test was performed with the Slidex Strepto Plus B kit (Biomérieux, France) following manufacturer's instructions, if a confirmation was needed.
After phenotypical processing, GBS positive samples were selected and Todd-Hewitt broth (Probac do Brasil®, Brazil) were sent in adequate conditions to LEMC for bacterial isolation, confirmatory identification, and serotyping. Samples were stored at −80 °C until molecular processing.
Molecular sample processingAt LEMC, samples were streaked onto blood agar. Isolates suggestive of S. agalactiae were recovered and confirmed as GBS by mass spectrometry (MALDI-TOF) using Microflex LT equipment (Bruker Daltonics/BD, Germany/USA). The analysis was performed in FlexAnalysis software (version 2.0; Bruker/Germany).19
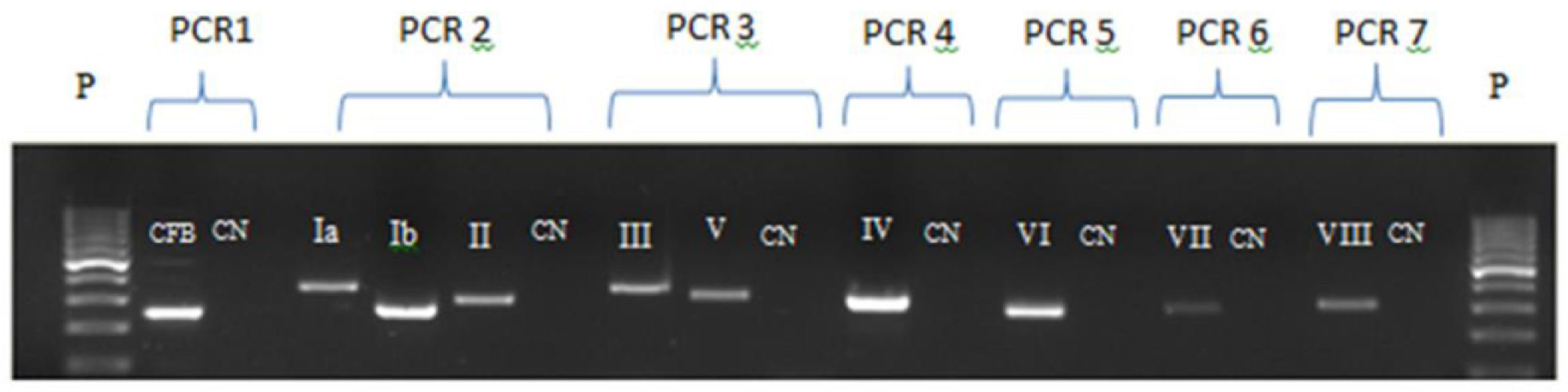
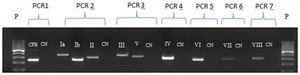
Bacterial DNA extraction was performed with Trizol/Chloroform-Brazol technique (LCG Biotechnology).20 Extracted DNA was stored at −20 °C until processing. The DNA extraction control was performed by Polimerase Chain Reaction (PCR) to detect the CAMP-factor gene of streptococci group B (cfb).21 Strain typing occurred only when PCR for cfb was positive, confirming bacterial DNA extraction (Fig. 1).
Electrophoresis gel for the seven PCRs for serotyping. P: molecular weight of 100 base pairs; CN: negative control; PCR 1: cfb extraction control; PCR 2: Capsular genotype Ia, Ib and II; PCR 3: Capsular genotype III and V; PCR 4: Capsular genotype IV; PCR 5: Capsular genotype VI; PCR 6: Capsular genotype VII and PCR 7: Capsular genotype VIII.
Capsular genotypes identification was performed through PCR amplification using specific primers (Ia - VIII), as reported by Kong et al. (2005).21 Reactions were standardized using a modified multiplex PCR proposed by Fiolo et al. (2012).22 Six different PCR techniques were performed for capsular genotyping: a multiplex PCR with capsular genotypes Ia, Ib and II primers; multiplex PCR for gene III and V; and four uniplex PCRs for each capsular genotype - IV, VI, VII and VIII (Fig. 1).
Primers’ specificity analysis was performed using specific control strains for capsular types Ia, Ib, II, III, IV, V, VI, VII and VIII.
Demographic and clinical dataData regarding demographic and gestational characteristics of GBS-positive pregnant women were retrieved from medical charts. The following variables were considered: age, parity, gestational age, presence or absence of fever, signs of chorioamnionitis, membranes rupture time, and history of a previous GBS colonized pregnancy.
Statistical analysisInitially, data were analyzed using a descriptive approach, through absolute and relative frequencies for categorical variables and summary measures for numerical variables. For the prevalence of each capsular genotype, 95% confidence intervals were also calculated.
Fisher's exact test was used to assess the association between capsular genotypes and several risk factors. Kruskal-Wallis non-parametric test was used to compare age and parity with genotype, due to the low frequency of some capsular genotypes. All statistical analyses were performed in SPSS 20.0 statistical software considering a significance level of 5%. Files are available as supplementary material.
ResultsA total of 8649 samples from at-risk pregnant women were collected during admission. Out of these, 820 GBS positive samples confirmed by phenotypical method were sent from maternity hospitals to LEMC. GBS recovery and confirmation by MALDI-TOF was possible for 42.9% (N = 352), proceeding to determination of the capsular genotype by PCR. Previous report regarding the prevalence of GBS carriage in pregnant women followed in the same maternity hospitals showed an estimate of 23%.23
Table 1 shows the distribution of observed capsular genotypes, as the estimated prevalence and its respective 95% confidence interval (CI). Out of 352 GBS isolates, the most frequently observed was genotype Ia (35.5%; 95%CI=30.5–40.8), followed by V (35.5%; 95%CI=29.1–39.3), II (11.6%; 95%CI=8.5–15.5), III (10.2%; 95%CI=7.3–13.9), Ib (6.5%; 95%CI=4.2–9.6), IV (1.1%; 95%CI=0.3–2.9), and VIII (0.3%; 95%CI=0.0–1.6).
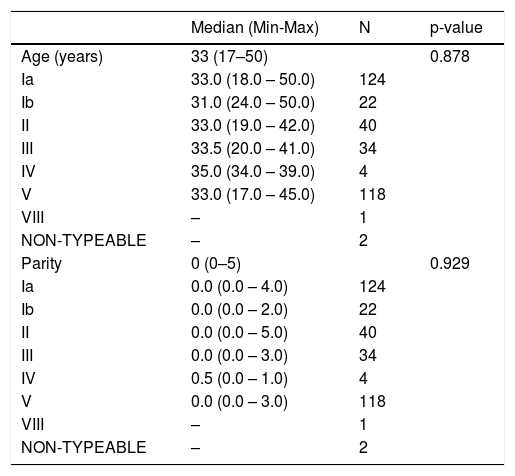
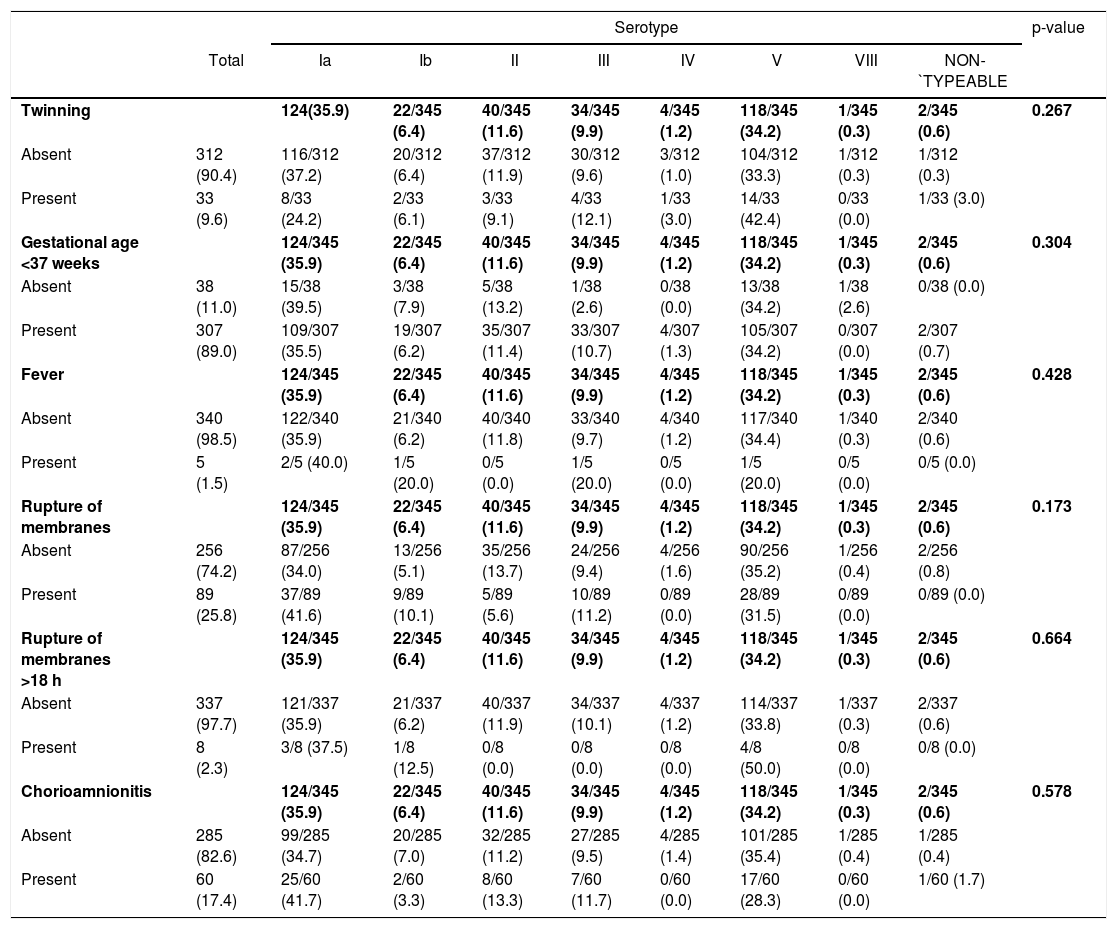
Among the 352 isolates, 345 records from the respective pregnant women were obtained and analyzed (136 from Maternidade ProMatre and 209 from Maternidade Santa Joana). Pregnant women's age varied between 17 and 50 years (mean= 32.4; SD=5.5). Regarding risk factors, 89.0% of the pregnant women had gestational age <37 weeks, 25.8% presented membranes rupture, and 17.4% had signs of chorioamnionitis. No cases with previous history of a GBS-infected child were seen.
Tables 2 and 3 show the analysis of association between capsular genotypes and demographic variables or risk factors. None of the analysis have shown significant differences between groups of exposure variables.
Summary measures of age and parity found in pregnant women colonized by GBS according to serotype (N = 345).
Distribution of GBS capsular genotypes according to the presence or absence of different risk factors in colonized pregnant women (N = 345).
This study was conducted aiming to evaluate the prevalence of different GBS capsular genotypes colonizing at-risk pregnant women in two private maternity hospitals in São Paulo, Brazil, over a 4-year period. In these hospitals a risk-based screening method is routinely performed to identify those who should receive intrapartum antibiotic prophylaxis according to the presence of any among the following intrapartum risk factors: delivery at <37 weeks’ gestation, intrapartum temperature ≥100.4° F (≥38.0 °C), or signs and symptoms of chorioamnionitis or rupture of membranes for ≥18 h.
The estimated prevalence of maternal colonization in these centers in a previous study conducted from 2007 to 2010 was 23%, similar to previous Brazilian data.23,24
Most prevalent capsular genotypes found in the present analysis were Ia and V, representing 35.5% and 34.1%, respectively. Previous studies analyzing GBS capsular genotype distribution among pregnant women in Brazil found different results.8,25 Dutra et al. (2014) conducted a multicenter study, covering all five Brazilian geographic regions, including GBS isolates from pregnant women, children and adults, and reported the following prevalences: Ia, 27.6%; Ib, 18.7%; II, 19.1%; III, 13.6%; IV, 8.1%; and V, 13.6%. When this pattern was compared across the regions, capsular genotype II was most frequently observed on south and southeast when compared to the other regions, while capsular genotype V was most frequent in northeast region.25 Botelho et al. (2018) have assessed the prevalence of different GBS capsular genotypes among pregnant women in a single public maternity hospital at Rio de Janeiro, Brazil. Capsular genotypes Ia (37.3%) and II (19.9%) were most frequently observed, followed by non-typeable (12.1%), Ib (11.1%), V (9.1%), III (6.8%) and IV (3.5%).8 Despite limited data provided by the present study involving a set of strains not representative of the country, strain diversity on several characteristics such as time, population evaluated, and typing technique is highlighted.
When the prevalence is assessed around the world, capsular genotype III is the most frequent, however types Ia, Ib, II, III and V represent 96% of total isolates.6
Several approaches have been used to reduce the incidence of invasive disease caused by GBS, such as the screening for colonized pregnant women including the use of intrapartum antibiotics, vaginal disinfection with chlorhexidine, and use of probiotics or GBS antagonist peptides.26 Although the use of intrapartum antibiotics has shown to be effective in reducing about 80% of early onset disease (EOD), this strategy is not able to prevent late onset disease (LOD) besides difficulties in implementation observed in many countries.27
In 2017, the World Health Organization (WHO) published recommendations listing desirable characteristics of future GBS vaccines, such as indication, target population (pregnant women in the second or third trimester of pregnancy), dose regimen, safety, efficacy, capsular genotype coverage, absence of adjuvants, immunogenicity, non-interference with other vaccines used during pregnancy, route of administration, cost-effectiveness data, among others. Regarding capsular genotypes, WHO recommends conjugated polysaccharide vaccines containing capsular genotypes Ia, Ib, II, III and V, although coverage failure may occur in some regions. It also emphasizes that the possibility of replacing capsular genotypes should be investigated.28 According to data shown in this study, the presence of capsular genotypes Ia and V in the formulation of future vaccines, would be able to cover 69.6% of the GBS capsular genotypes colonizing pregnant women in São Paulo, Brazil.
Currently, no vaccine exists for preventing GBS disease, but maternal immunization with multiple capsular genotypes of protein-conjugated GBS capsular polysaccharides may reduce the disease risk in neonates and young infants through transplacental passage of protective immunoglobulins. The characterization of serotype-specific distribution, especially in some geographical areas, is crucial to guide vaccine composition in terms of serotype diversity coverage. Different published data are available for monovalent, bivalent, trivalent and hexavalent GBS vaccines evaluated in non-pregnant and pregnant women. Investigational monovalent and bivalent GBS vaccines containing capsular polysaccharides of capsular genotypes Ia, Ib, II, III, or V conjugated to tetanus toxoid using various doses have been evaluated and studied in non-pregnant, healthy volunteers. A trivalent (Ia, Ib, and III) GBS capsular polysaccharide conjugate vaccine has also been evaluated in clinical trials of non-pregnant and pregnant women. These studies showed an acceptable safety profile of GBS polysaccharide conjugate vaccines administered to pregnant women, as well as the induction of immune responses to the GBS vaccine capsular genotypes that resulted in transplacental transfer of antibodies to their infants. Data from a phase 1/2 study suggest that three doses (5 μg, 10 μg, and 20 μg) of the first hexavalent GBS vaccine are safe and well tolerated and induce immune responses to six GBS capsular genotypes lasting at least six months after vaccination in healthy men and non-pregnant women aged 18–49 years.29
As secondary objective, the relationship between GBS capsular genotypes and gestational and fetal outcomes showed no significant differences. To the authors’ knowledge, data on this relationship have not been previously assessed. A better comprehension of this relationship is important regarding the adoption of more specific treatment strategies. Edwards et al. (2019) found a decreased risk of short cervix, chorioamnionitis, wound infection, operative delivery, and birth at <34 and <37 weeks gestation among GBS colonized women when compared to negative ones. Risk reduction in the sample is attributed to the administration of prophylactic intravenous antibiotics during labor as a routine.30
Although these important findings, our study has one limitation related to data extrapolation. Since the studied population was obtained in private healthcare services in a single city, the capsular genotypes distribution may not be representative of the whole country. In addition, considering that study sample was obtained in a private healthcare service, the socio-economic background of the pregnant women may have influenced colonization rates observed.
ConclusionGBS capsular genotype Ia is the most prevalent among at-risk pregnant women in a complex of private maternity hospitals in São Paulo, followed by V, II, III and Ib, which together correspond to 97.9% of the isolates. When the relation between capsular genotype and gestational and fetal outcomes were assessed, no statistically significant differences were observed.
The presence of capsular genotypes Ia and V in the formulation of candidate vaccines would cover 69.6% of capsular genotypes colonizing pregnant women in the studied population. This highlights the need for further investments and studies in this subject.
We thank Dr. Sérgio Eduardo Longo Fracalanzza (Instituto de Microbiologia Paulo de Góes da Universidade Federal do Rio de Janeiro - UFRJ) for having kindly given specific control strains for capsular types Ia, Ib, II, III, IV, V, VI, VII and VIII, and SENSE Company for the support with medical writing during the development of this manuscript. The study was funded by the Foundation for Research Support of the São Paulo State (Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP), under the process number 2016/04708–6.