Chronic hepatitis B is an important health problem that can progress to cirrhosis and complications such as hepatocellular carcinoma. There is approximately 290 million of people with chronic hepatitis B virus (HBV) infection worldwide, however only 10% of patients are currently identified.
Most part of Brazil is considered of low prevalence of HBV infection but there are some regions with higher frequency of carriers. Unfortunately, many infected patients are not yet identified nor evaluated for treatment.
The Brazilian Society of Infectious Diseases (SBI) and the Brazilian Society of Hepatology worked together to elaborate a guideline for diagnosis and treatment of hepatitis B. The document includes information regarding the population to be tested, diagnostic tools, indications of treatment, therapeutic schemes and also how to handle HBV infection in specific situations (pregnancy, children, immunosuppression, etc).
Delta infection is also part of the guideline, since it is an important infection in some parts of the country.
Hepatitis B is an important public health problem worldwide, with almost 300 million of individuals infected with HBV. In 2016 the World Health Organization (WHO) established the goal of eliminating viral hepatitis B and C as a public health problem by the 2030. For this reason, all countries should be involved in improving diagnosis and treatment of these infections. Brazil is one of the countries signatories of the WHO aims for elimination of viral hepatitis and the present recommendations may help physicians and public authorities to reach the established goals for 2030.
The recommendations were developed by a panel of experts chosen by The Brazilian Society of Hepatology (SBH) and The Brazilian Society of Infectious Disease (SBI) based on evidence from the literature and on the experts’ experience.
The evidence and recommendations have been graded according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) system adapted by the European Association of Study of Liver (EASL). The strength of recommendations (strong: 1, weak: 2) was based on the quality (grade) of evidence (I, II-1, II-2, II-3, III), as stated below:
Grade evidence
I Randomized, controlled trials
II-1 Controlled trials without randomization
II-2 Cohort or case-control analytical studies
II-3 Multiple time series, dramatic uncontrolled experiments
III Opinions of respected authorities, descriptive epidemiology
Grade recommendation
- 1
Strong recommendation: Factors influencing the strength of the recommendation included quality of the evidence, presumed patient-important outcomes, and cost;
- 2
Weaker recommendation: Variability in preferences and values, or more uncertainty: more likely a weak recommendation is warranted. Recommendation is made with less certainty: higher cost or resource consumption.
References
- 1
European-Union-HCV-Collaborators. Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. The lancet Gastroenterology & hepatology. 2017;2(5):325–36.
- 2
European Association fot the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67: 370–398
Chronic hepatitis B is an important health problem that can progress to cirrhosis and complications such as hepatocellular carcinoma. There is approximately 300 million of people with chronic hepatitis B virus (HBV) infection worldwide, however only 10% of patients are currently identified.
The importance of the screening is to identify HBV carriers in order to evaluate those for whom treatment is indicated, since antiviral treatment is available and inhibit viral replication in almost all cases, and also identify those who are susceptible (negativity for all HBV markers), with indication of vaccination.
Other advantage of HBV screening is to prevent perinatal transmission in pregnant women, domiciliary and sexual transmission by chronic carriers and to identify patients with past infection and possible risk of reactivation under immunosuppression, chemotherapy, or immunobiological use.
Hepatitis B fulfills the criteria for screening: there is a safe and validated serological test for diagnosis, at low cost and widely available. The following high-risk populations are priority for screening:
1) individuals with liver disease or elevated aminotransferases; 2) relatives, household contacts, infants and sexual partners of HBV-carriers; 3) individuals who require immunosuppressive therapy, chemotherapy, or immunobiological use; 4) users of injectable drugs or other illicit drugs; 5) individuals receiving or with a past history of unsafe injections (potentially contaminated syringes or needles); 6) men who have sex with men, individuals with multiple sex partners without the use of condoms and with sexually transmitted diseases (STDs), sex workers, transgenders, and individuals in serodifferent sexual relationships; 7) inmates of correctional facilities or people deprived of their liberty; 8) dialysis patients; 9) HCV- or HIV-infected individuals; 10) pregnant women and children born to HBsAg-positive mothers; 11) health professionals or professionals exposed to contaminated biological material; 12) blood or organ/tissue donors; 13) individuals born or residents in regions with high or intermediate HBV endemicity (prevalence of HBsAg > 2%); 14) residents and staff of facilities for people with developmental disabilities; 15) travelers to countries or locations with an intermediate or high prevalence of HBV infection; 16) homeless people, and 17) non-vaccinated individuals with diabetes.
It should be underscored that individuals classified as susceptible after hepatitis B screening must be referred for immunization.
International guidelines diverge on which tests to use for hepatitis B screening. Most guidelines recommend serological testing for HBsAg and anti-HBs. Alternatively, total anti-HBc can be used for screening given that those individuals who are positive are subsequently tested for HBsAg and anti-HBs to differentiate current infection from past exposure to HBV.
In HIV-infected patients, in candidates to immunosuppressive therapy, chemotherapy and treatment for HCV, with risk of infection reactivation even in HBsAg negative patients, the screening should be done with HBsAg and anti-HBc. Anti-HBc positive patients have to complete the investigation with HBV-DNA determination. HBV-DNA detectable in HBsAg-negative patients identify those at risk for viral reactivation in situations of immunosuppression.
Recommendations
Screening of HBV infection is indicated for all pregnant women, blood donors, and patients with any identified risk factor (level II-1 evidence, recommendation 1).
Patients with no HBV markers should be oriented to vaccination (level I evidence, recommendation 1).
Screening should be performed using HBsAg and anti-HBs. In patients living with HIV and those who will be submitted to immunosuppression, total anti-HBc should also be investigated (level II-1 evidence, recommendation 1).
In patients with isolated anti-HBc serology, occult infection with HBV should be considered (level II-2 evidence, recommendation 1)
References
- 1
Asian Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98.
- 2
EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-98.
- 3
Terrault NA et al. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatol 2018;67:1560- 99.
- 4
Robotin MC & George J. Community-based hepatitis B screening: what works? Hepatol Int 2014;8:478–492.
- 5
Wang Q et al. Significance of anti-HBc alone serological status in clinical practice. Lancet Gastroenterol Hepatol 2017; 2:123-34.
- 6
Joller-Jamelka HI et al. Detection of HBs antigen in “anti-HBc alone” positive sera J Hepatol 1994;21:269-272.
In Brazil, the hepatitis B vaccine was introduced in the National Immunization Calendar in the 1990s and was indicated for all children in the first year of life. In 2016, vaccine coverage was expanded and is now offered to all individuals regardless of age.
In healthy individuals, hepatitis B vaccination with the standard dose (20 μg) at 0, 1 and 6 months provides a protective serum response (anti-HBs > 10 mIU/mL) in more than 90% of adults and in 95% of infants and children. Modified schedules or doses, including doubling the standard antigen dose or administering additional doses, can increase the response rates. However, data on these alternative vaccination regimens are limited.
In hemodialysis patients or patients with chronic kidney disease, the median protection rate is 60.1% among those with diabetes mellitus versus 75.1% among those without diabetes. For hepatitis B vaccination of adult hemodialysis patients, a high dose (40 μg) of Recombivax HB®, administered at 0, 1 and 6 months, or a high dose (40 μg) of Engerix-B®, administered at 0, 1, 2 and 6 months, is recommended.
Alternative vaccination schedules (for example, 0, 1 and 4 months or 0, 2 and 4 months) have been shown to elicit dose-specific and final seroprotection rates similar to those obtained with the 0, 1 and 6-month schedule. An increased interval between the first two doses has little effect on immunogenicity or on the final antibody titer. The third dose confers the maximum level of seroprotection as well as long-term protection. Longer intervals between the last two doses (for example, 11 months) result in higher final antibody levels but can increase the risk of acquiring HBV infection in people with a delayed response to vaccination. Higher geometric mean titers are associated with long-term persistence of measurable anti-HBs. The Twinrix® vaccine can be administered before travel to countries with intermediate or high prevalence of HBV infection or before any other potential exposure using an accelerated schedule on days 0, 7 and 21–30, followed by a mandatory dose at 12 months. Recently, the HepB-CpG (Heplisav-B®) vaccine was approved for clinical use in the United States. This vaccine uses a new adjuvant and two doses (0 and 1 month) and is indicated for people older than 18 years without other restrictions.
The guidelines of the Ministry of Health recommend the use of alternative regimens (high dose of 40 μg; 0, 1, 2 and 6–12 months) for solid organ transplant recipients, patients with cancer, patients who require chemotherapy, radiotherapy and corticotherapy, patients with other immunodeficiencies, and for pre-dialysis or dialysis patients with chronic kidney disease.
Routine serological testing for the detection of hepatitis B immunity is not necessary after routine vaccination of infants, children or adults because of the high efficacy of the vaccine. However, testing for HBsAg and evaluation of protective anti-HBs levels after vaccination is recommended in situations in which this information will be important for subsequent clinical management (revaccination or other types of protection against hepatitis B such as hyperimmune globulin), namely:
- -
Babies born to HBsAg-positive mothers or to mothers with unknown serological status;
- -
Healthcare and public safety workers at risk of exposure to blood and body fluids;
- -
Patients on hemodialysis or peritoneal dialysis and people living with HIV and other immunocompromized individuals (recipients of hematopoietic stem cell transplants or patients undergoing chemotherapy) to determine the need for revaccination and the type of testing during follow-up;
- -
HBsAg-serodifferent sexual partners;
- -
Men having sex with men, sex workers, transsexual people, individuals with sexually transmitted infections, individuals at risk of unprotected sexual exposure;
- -
Chemically dependent people;
- -
People deprived of their liberty;
- -
Household contacts of chronic hepatitis B carriers;
- -
Patients with chronic liver disease.
- -
According to the guidelines of the Ministry of Health, vaccinated individuals belonging to the key-populations described above who do not respond with adequate antibody levels should be revaccinated using an additional 3-dose series of the vaccine. Individuals who remain anti-HBs negative after two complete regimens with three doses must be considered non-responders and susceptible when exposed. The use of hyperimmune globulin is recommended in this case.
Several possible strategies have been proposed in the literature for hepatitis B vaccine non-responders, but the scientific evidence is controversial:
- -
Revaccination or increase of the vaccine dose;
- -
Intradermal vaccination;
- -
New adjuvants (e.g., Heplisav-B®);
- -
Increased immunogenicity (e.g., protein PreS1 and PreS2);
- -
Therapeutic supplementation (e.g., GM-CSF, levamisole, praziquantel).
Recommendations
- 1
Hepatitis B vaccination is part of the National Immunization Calendar for all children at birth and is available for all individuals regardless of age (level I evidence, recommendation 1).
- 2
Alternative vaccination regimens using a larger number of doses or a higher dose are indicated for hemodialysis patients, solid organ transplant recipients, patients with cancer, patients who require chemotherapy, radiotherapy and corticotherapy, and patients with other immunodeficiencies (level II-1 evidence, recommendation 1).
- 3
Testing for anti-HBs after vaccination is not indicated for all individuals but is recommended only for groups of patients with an indication for revaccination (patients continuously exposed to HBV). Revaccination should consist of an additional 3-dose series of the vaccine (level II-2 evidence, recommendation 1).
References
- 1
Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Manual dos Centros de Referência para Imunobiológicos Especiais / Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis. – 4. ed. – Brasília: Ministério da Saúde, 2014. 160 p.: il.
- 2
Das S, Ramakrishnan K, Behera SK, Ganesapandian M, Xavier AS, Selvarajan S. Hepatitis B vaccine and immunoglobulin: Key concepts. J Clin Transl Hepatol 2019;7(2):165–171.
- 3
Van Den Ende Ca, Marano Cb, Van Ahee Aa The immunogenicity and safety of GSK’s recombinant hepatitis B vaccine in adults: a systematic review of 30 years of experience EXPERT REVIEW OF VACCINES, 2017 VOL. 16, NO. 8, 811–832
- 4
Rubin LG, Levin MJ, Ljungman P, et al.; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014;58:e44–100.
- 5
Fabrizi F, Dixit V, Messa P, Martin P. Hepatitis B virus vaccine in chronic kidney disease: improved immunogenicity by adjuvants? A meta-analysis of randomized trials. Vaccine 2012; 30: 2295–300.
- 6
Hadler SC, de Monzon MA, Lugo DR, Perez M. Effect of timing of hepatitis B vaccine doses on response to vaccine in Yucpa Indians. Vaccine 1989; 7: 106–10.
- 7
Jilg W, Schmidt M, Deinhardt F. Vaccination against hepatitis B: comparison of three different vaccination schedules. J Infect Dis 1989; 160: 766–9.
- 8
Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018; 67(1):1-31.
- 9
Tara Vinyette Saco, Alexandra T. Strauss, Dennis K. Ledford, Hepatitis B vaccine non-responders: possible mechanisms and solutions, Annals of Allergy, Asthma & Immunology (2018).
The diagnostic tests used in clinical practice for patients with hepatitis B are the detection of viral antigens (HBsAg and HBeAg), antibodies (anti-HBc IgM or IgG, anti-HBe and anti-HBs), and quantification of viral load (HBV-DNA).
However, more recently the quantification of HBsAg (qHBsAg), reflecting intra-hepatic activity of covalently closed circular DNA (ccc-DNA), has become an useful tool for the comprehension of the natural history of the disease, as well to help in therapeutic decisions. There are some commercial tests approved for this purpose but they are not broadly available in Brazil.
HBsAg quantification is useful to: diagnosis of chronic infection phases; risk of disease progression; chance of response to interferon therapy; and, in the treatment with antiviral analogs, to evaluate the possibility of stopping therapy after a consolidation period.
The levels of qHBsAg differ between phases of chronic infection as well as among genotypes. Higher levels (> 4 log UI/mL) are seen in immunotolerant phase (HBeAg positive, absence of activity and high viral load) and lower levels in inactive carries (HBeAg negative, absence of activity and viral load < 2000 UI/mL). In patients infected with genotype A, qHBsAg levels are higher than in other genotypes.
In HBeAg negative patients, qHBsAg < 1000 UI/mL and HBV-DNA < 2000 UI/mL indicate the presence of inactive chronic infection and among these patients qHBsAg < 100 UI/mL is associate with spontaneous clearance of HBsAg.
Determination of qHBsAg is also useful to determine the frequency of ALT and elastography monitoring in patients with inactive infection (HBeAg negative). Levels of qHBsAg < 1000 UI/mL suggest annual ALT and HBV-DNA assessment and triennial fibrosis evaluation; with qHBsAg ≥ 1000 UI/mL these tests should be done every six months and fibrosis evaluation every two years.
Quantification of HBsAg has also been used to predict the risk of HCC in HBeAg negative patients with low viral load. Among Asiatics the risk was 14-fold higher in those with qHBsAg > 1000 vs < 1000 UI/mL.
During antiviral therapy with interferon, HBsAg is very useful as a stopping rule in HBeAg-positive patients. Patients with genotype D, with no or little reduction of quantitative HBsAg, should discontinue treatment, while patients with genotype A should reduce quantitative HBsAg by more than 1 log10 to continue treatment.
It is important to remember that the relevance of qHBsAg is related to HBV genotypes and has assessed for A, B, C and D genotypes. However it has not been validated for genotype F, frequent in hyperendemic areas in Brazil.
Regarding genotypes, the phylogenetic HBV analysis shows the existence of 10 distinct genotypes (A–J). In Brazil genotypes A, D and F are the most prevalent. The usefulness of HBV genotyping is much less evident when compared to hepatitis C. Although the HBV genotype has implications for the natural history of the disease and the chance of responding to interferon treatment, genotype is of little importance for the treatment with nucleotide or nucleoside analogs. A higher chance of response in patients treated with IFN, with HBeAg seroconversion and HBV-DNA < 2000 UI six months after ending treatment is observed in infections caused by genotype A while the lowest chance is seen with genotype D infections. Specifically for genotype F, prevalent in endemic areas in Brazil, the relation with therapeutic response to IFN is still undetermined.
Indication for determining antiviral resistance varies according to the situation. Antiviral resistance detected before treatment is considered to be primary. There are few studies in the literature evaluating HBV-naive patients with primary resistance, but the numbers vary widely according to geographic region. In some regions of China and Korea the prevalence of primary resistance to entecavir varies from 4 to 60% associated to a mutation in polymerase gene. In Brazil, there are few studies evaluating this question, with emphasis to the study from Pacheco et al. (2018), with 189 patients, showing 6% prevalence in the Amazon region, mostly infected with genotype A and F, while in Northeast no cases of resistance were found.
The other situation is the emergence of resistance during HBV antiviral treatment. Nowadays, with the use of ETV, TDF and TAF as first line drugs, therapeutic failure due to drug resistance has become a rare event. The diagnosis of resistance is done in patients receiving antiviral therapy who present viral load elevation of 1 log or greater compared to basal level after an initial virological response and confirmation of adherence; this situation is characterized as virological breakthrough, usually associated with antiviral drug resistance.
The diagnostic test to detect antiviral resistance include restriction fragment polymorphism analysis (RFLP), hybridization and sequencing. To be performed these tests need a viral load of at least 1000 UI/mL in the sample.
Recommendations
- 1
Quantitative HBsAg is useful for the evaluation of HBeAg negative patients, as a support for the characterization of the chronic phases of infection and to help in the decision of stopping treatment. (level II-1 evidence, recommendation 2).
- 2
Quantitative HBsAg is useful for HBeAg-positive patients treated with interferon as a stopping rule (level I evidence, recommendation 2).
- 3
The indication of genotyping for hepatitis B treatment is only justified for HBeAg-positive patients who can be treated with interferon. This option also includes the availability of quantitative HBsAg since interdependence exists in the interpretation of the two tests (level II-1 evidence, recommendation 2).
- 4
Routine investigation of antiviral resistance is not justified for definition of the therapeutic strategy, especially in patients for whom the use of TDF is defined. (level II-2 evidence, recommendation 2).
- 5
Antiviral resistance determination can be useful in adherent antiviral treated patients presenting viral load elevation after an initial response (level II-1 evidence, recommendation 2).
References
- 1
Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HLA, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66(2):398-411
- 2
Lin CL, Kao JH. Natural history of acute and chronic hepatitis B: The role of HBV genotypes and mutants. Best Pract Res Clin Gastroenterol. 2017 ;31(3):249-255.
- 3
Enomoto M, Tamori A, Nishiguchi S. Hepatitis B virus genotypes and response to antiviral therapy. Clinical Laboratory. 2005; 52(1-2):43-47.
- 4
Gomes-Gouvêa MS, Ferreira AC, Teixeira R, Andrade JR, Ferreira AS, Barros LM, et al. HBV carrying drug-resistance mutations in chronically infected treatment-naive patients. Antivir Ther. 2015;20(4):387-95.
- 5
Pacheco SR, Dos Santos MIMA, Stocker A, Zarife MAS, Schinoni MI, Paraná R, Dos Reis MG, Silva LK. Genotyping of HBV and tracking of resistance mutations in treatment-naïve patients with chronic hepatitis B. Infect Drug Resist. 2017;10:201-207.
A few years ago, entecavir (nucleoside analog) and tenofovir (nucleotide analog) were added to the Ministry of Health’s Clinical Protocol and Therapeutic Guidelines for the treatment of chronic hepatitis B since these two drugs showed the lowest incidence of resistance, i.e., the highest genetic barrier, when compared to drugs previously used in Brazil such as lamivudine and adefovir. The criteria for using one or the other drug are different. In Brazil, patients with cirrhosis and other comorbidities like type-2 diabetes or high blood pressure are usually candidates to receive entecavir. Therefore, even if the entire cohort of patients with HBV treated within the Brazilian National Health System (SUS) were evaluated, we would not have a homogeneous group of patients to compare the efficacy and safety of the two drugs.
Registry-based studies of entecavir and tenofovir evaluated alanine aminotransferase (ALT) normalization, seroconversion of HBeAg (21% vs 21%) and HBsAg negativity, as well as the rate of undetectable HBV DNA at 48 and 96 weeks of treatment (67% and 76% in HBeAg-positive patients and 90% and 93% in HBeAg-negative patients, respectively). In the two studies, a liver biopsy was obtained from the patients at baseline and one and five years after treatment. Significant improvement in the degree of inflammation according to the Ishak score was observed in the first year of treatment, while there was little improvement in the degree of hepatic fibrosis. However, in addition to important improvement in inflammatory parameters, biopsies obtained five and six years after treatment also exhibited significant reduction in fibrosis. There were even reports of patients with cirrhosis at baseline biopsy who progressed to complete regression of fibrosis after five years.
A meta-analysis published in 2017 evaluated and compared the efficacy and safety of tenofovir and entecavir for the treatment of patients with chronic hepatitis and/or cirrhosis due to HBV. The authors reported a significant difference in ALT normalization at three and six months favoring tenofovir, but not after six months, as well as in the rate of undetectable HBV DNA in the third month of treatment, but not after that period. In patients with cirrhosis, no difference in the control of viremia or improvement of liver function was observed between the two drugs. Patients treated with tenofovir exhibited greater changes in glomerular filtration rate and hypophosphatemia incidence than those receiving entecavir.
Tenofovir alafenamide (TAF) is a new tenofovir pro-drug recently approved in Brazil that reduces plasma tenofovir levels by 90% compared to tenofovir disoproxil fumarate (TDF), thereby decreasing the loss of bone mineral density and renal toxicity. An open prospective study investigated 75 patients with chronic hepatitis B using 300 mg/day tenofovir for at least 12 months, with undetectable HBV DNA, who were switched to 25 mg/day TAF and followed up for 24 weeks. After the switch, a significant decrease was observed in urinary beta-2-microglobulin/creatinine and urinary retinol-binding protein/creatinine ratios at week 12 (p < 0.01 for both). There was no change in the mean glomerular filtration rate but tubular reabsorption of phosphate was reduced at week 24 (p < 0.05). The authors concluded that patients using TDF who are switched to TAF have significant improvement in bone mineral density and in some renal tubular function parameters as early as after 12 weeks of treatment with TAF. Compared to TDF, TAF exhibited the same efficacy in terms of undetectability of HBV-DNA and more frequent ALT normalization and in two registry-based studies.
Recommendations
- 1
Pegylated interferon, entecavir, TDF and TAF are drugs approved for the treatment of hepatitis B. The oral antivirals (ETV, TDF or TAF) are equally effective in patients with treatment indication (level II-2 evidence, recommendation 1).
- 2
In patients with evidence or higher risk of renal or bone alterations, entecavir and TAF are the most indicated drugs (level II-1 evidence, recommendation 1).
References
- 1
Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, Zhang H, Tenney DJ, Tamez R, Iloeje U. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51(2):422-30.
- 2
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468-75.
- 3
Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and Meta-analysis. Int Immunopharmacol. 2017;42:168-175.
- 4
Henry L Y Chan, Scott Fung, Wai Kay Seto, Wan-Long Chuang, Chi-Yi Chen, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016; 1: 185–95.
- 5
Maria Buti, Edward Gane, Wai Kay Seto, Henry L Y Chan, Wan-Long Chuang, Tatjana Stepanova, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016; 1: 196–206.
- 6
Viganò M, Loglio A, Labanca S, Zaltron S, Castelli F, Andreone P, Messina V, Ganga R, Coppola N, Marrone A, Russello M, Marzano A, Tucci A, Taliani G, Fasano M, Fagiuoli S, Villa E, Bronte F, Santantonio T, Brancaccio G, Occhipinti V, Facchetti F, Grossi G, Rumi M, Lampertico P. Effectiveness and safety of switching to entecavir hepatitis B patients developing kidney dysfunction during tenofovir. Liver Int. 2019;39(3):484-493.
It is estimated that 240 million people worldwide are chronic carriers of HBV; however, about two billion people show serological evidence of past infection, demonstrating that about 90% eliminated the virus during the phase of acute infection. Most cases are asymptomatic and less than 40% have the classical icteric form. About 1% of cases may develop fulminant hepatitis, a condition with high mortality rate.
In general, due to universal vaccination, the incidence of acute hepatitis has dropped in the world and in Brazil, especially in the younger population. A higher frequency is observed in older adults whose immune status can modify the natural history of the infection. Since most immunocompetent adults eliminate the virus spontaneously, drug treatment is not indicated. There is no consensus as to whether cases with signs of severe disease would benefit from the antiviral drugs recommended for chronic infection, or whether treatment can prevent progression to chronic infection in individuals with immune deficiencies. It has also been questioned whether the use of antiviral drugs in severe forms would, by lowering the viral load, reduces the individual’s immune response, preventing clearance of the virus.
Regarding the treatment of severe acute hepatitis B, although randomized controlled trials are available, including cases with a diagnosis of acute liver failure (fulminant), those studies were unable to show superiority in the prevention of death or need for liver transplantation in the treated group. In addition, the studies were conducted with an insufficient number of patients and heterogeneous samples.
In addition to randomized controlled trials, there are many case series with or without historical controls that show lower mortality and lower rates of progression to transplant among patients with a diagnosis of severe acute hepatitis (INR > 1.5, total bilirubin > 10 mg/dL) or acute liver failure (fulminant) treated with antiviral drugs (lamivudine, tenofovir, or entecavir). The best results were obtained when treatment was initiated early. Individuals with long-term disease (more than two months) also benefitted from drug treatment.
As for the prevention of chronic infection by antiviral treatment during the acute phase of hepatitis, the controversies are even greater. Few studies reported a higher risk of progression to chronic infection in individuals with some immunodeficiency after acute HBV infection, except some case reports, and the evidence of higher prevalence of chronic infection in some specific groups such as HIV carriers, patients with chronic kidney disease and older adults. Furthermore, there are also no studies using drug treatment to prevent the progression to chronicity.
Most international guidelines (AASLD, EASL, Asian-Pacific) recommend treatment in specific cases, considering experiences described in the literature.
Recommendations
- 1
To treat with nucleos(t)ide analogs (tenofovir, TAF or entecavir) patients with a diagnosis of acute liver failure, patients with severe acute hepatitis (INR > 1.5) and patients who have protracted course for more than 2 months (level II-3 evidence, recommendation 1).
- 2
To consider treating patients with acute hepatitis B and some degree of immunodeficiency, including older adults (level III evidence, recommendation 2).
References
- 1
Asian-Pacific Clinical Practice Guidelines on the management of hepatitis B: a 2015 update. Hepatol Int (2016) 10:1-98
- 2
EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of Hepatology, 2017: 67:370-98
- 3
Terrault N, Lok AS, McMahon BJ. Update on Prevention, Diagnosis and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology, 2018:67:1560-99.
- 4
Pharmacological Interventions for Acute Hepatitis B. Cochrane Database of Systematic Reviews, 2017 (3). CD011645. doi: 10.1002/14651858.CD011645
- 5
Tillman HL et al. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. J Viral Hepat 2006 Apr;13(4):256-63
- 6
McKeating C et al. Progression from acute to chronic hepatitis B is more common in older adults. Ulster Med J 2018:87:177-80.
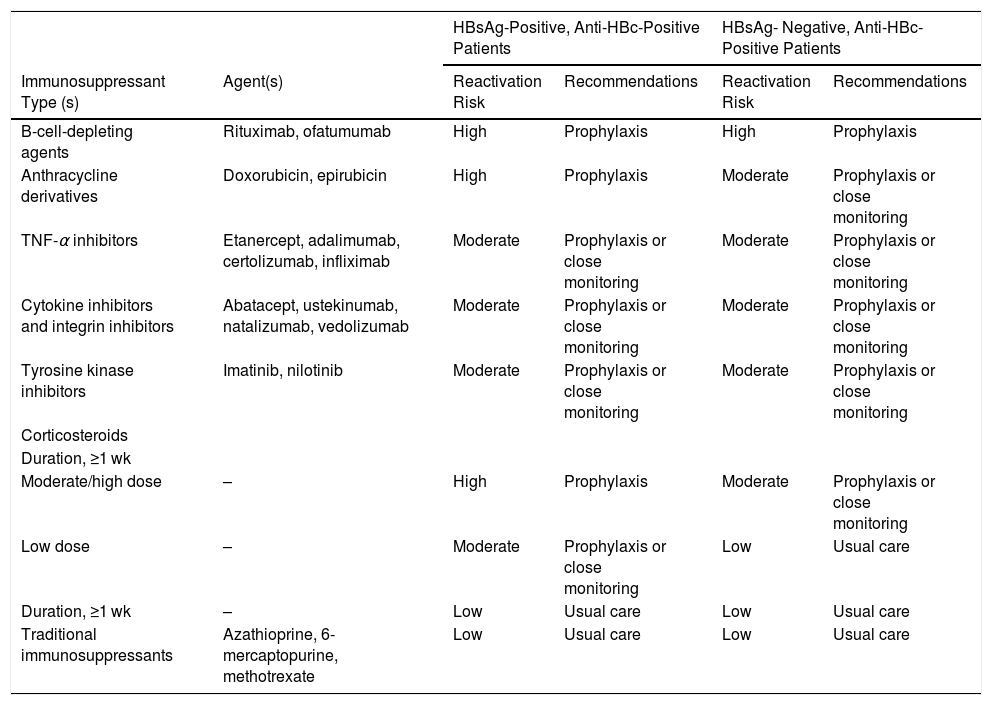
Several clinical conditions can be associated with reactivation of HBV infection. The risk depends on the patient’s serology (HBsAg positive or HBsAg negative/anti-HBc positive) and type of immunosuppression. In case of use of immunosuppressive or immunobiological drugs, the risk is higher for patients receiving chemotherapy; immunosuppression for the treatment of solid tumors and solid organ or stem cell transplantation; immunosuppression in hematological neoplasias (lymphoma, leukemias), rheumatological (rheumatoid arthritis, psoriasis), and gastrointestinal diseases (Crohn, ulcerative colitis; biological therapy with monoclonal antibodies - anti-CD20 [rituximab] and anti-CD52 [ofatumumab]), anthracyclines (doxorubicin/TACE), calcineurin inhibitors (cyclosporin, tacrolimus), and tyrosine kinase inhibitors (imatinib); cytokine-based therapies (abatacept, mogamulizumab, natalizumab, and vedolizumab), and high-dose corticosteroids for more than four weeks (Table 1).
Preventive antiviral therapy includes prophylactic therapy (before the occurrence of viremia) and preemptive treatment after the occurrence of viremia but still during the asymptomatic phase. In patients at high risk (> 10%) of viral reactivation, antiviral prophylaxis should be initiated before beginning immunosuppression or chemotherapy. In patients at moderate risk (1–10%) of viral reactivation, prophylactic treatment can be initiated or, alternatively, careful monitoring of HBV DNA for early identification of viremia onset and initiation of preemptive therapy. Patients at low risk (< 1%) of viral reactivation do not require prophylactic therapy (see the risk classification table at the end of this section).
In patients receiving antiviral prophylaxis, liver function and HBV DNA levels should be evaluated every 3–6 months during immunosuppression/chemotherapy. In patients without an indication for antiviral prophylaxis, HBV DNA and ALT should be monitored every 1–3 months. Additional investigation of HBsAg is recommended in patients with an HBsAg(-)/anti-HBc(+) serological profile since, if HBsAg becomes positive, initiation of preemptive therapy is indicated. HBV DNA monitoring should be extended to 12 months after the end of treatment because of the possibility of late reactivation.
If indicated, antiviral prophylaxis should be initiated at least one week before or simultaneously with immunosuppressive therapy. Antiviral prophylaxis should be maintained after ending immunosuppression for at least six months, or for 12 months in case of anti-CD20 therapy. Drugs with a high genetic barrier to resistance are indicated as first-line treatment for preventing viral reactivation: entecavir, tenofovir disoproxil, and tenofovir alafenamide.
Recommendations
- 1
All candidates for chemotherapy or immunosuppressive therapy should undergo serological testing for hepatitis B (mandatory HBsAg and anti-HBc) (level I evidence, recommendation 1).
- 2
Prophylaxis of reactivation should be performed based on the risk stratification shown in the attached table (level II-2 evidence, recommendation 1).
- 3
Entecavir or tenofovir should be used for prophylaxis and should be maintained for 6 months (12–18 months in the case of rituximab) after discontinuation of the immunosuppressive or immunobiological agent (level II-2 evidence, recommendation 1).
Table 1. 2015 American Gastroenterological Association Guidelines on the Risk for and Prevention of Hepatitis B Virus (HBV) Reactivation, by Immunosuppressive Agent and HBV Surface Antigen (HBsAg) and Antibody to HBV Core Antigen (Anti-HBc) Status.
| HBsAg-Positive, Anti-HBc-Positive Patients | HBsAg- Negative, Anti-HBc-Positive Patients | ||||
|---|---|---|---|---|---|
| Immunosuppressant Type (s) | Agent(s) | Reactivation Risk | Recommendations | Reactivation Risk | Recommendations |
| B-cell-depleting agents | Rituximab, ofatumumab | High | Prophylaxis | High | Prophylaxis |
| Anthracycline derivatives | Doxorubicin, epirubicin | High | Prophylaxis | Moderate | Prophylaxis or close monitoring |
| TNF-α inhibitors | Etanercept, adalimumab, certolizumab, infliximab | Moderate | Prophylaxis or close monitoring | Moderate | Prophylaxis or close monitoring |
| Cytokine inhibitors and integrin inhibitors | Abatacept, ustekinumab, natalizumab, vedolizumab | Moderate | Prophylaxis or close monitoring | Moderate | Prophylaxis or close monitoring |
| Tyrosine kinase inhibitors | Imatinib, nilotinib | Moderate | Prophylaxis or close monitoring | Moderate | Prophylaxis or close monitoring |
| Corticosteroids | |||||
| Duration, ≥1 wk | |||||
| Moderate/high dose | – | High | Prophylaxis | Moderate | Prophylaxis or close monitoring |
| Low dose | – | Moderate | Prophylaxis or close monitoring | Low | Usual care |
| Duration, ≥1 wk | – | Low | Usual care | Low | Usual care |
| Traditional immunosuppressants | Azathioprine, 6-mercaptopurine, methotrexate | Low | Usual care | Low | Usual care |
Data are from [34].
aTotal daily dose of prednisone (or equivalent): low dose, <10 mg; moderate dose, 10–20 mg; high dose, >20 mg.
References
- 1
Terrault N, Lok AS, McMahon BJ et al. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: 2018 Hepatitis B Guidance. Hepatology, 67(4):1560-1647.
- 2
Min-Yue Zhang et al., Systematic review with network meta-analysis: Comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget, vol.7, No.21, 2016: 30642-58
- 3
Jessica Hwang, and Anna S.-F. Lok. Incidence of hepatitis B virus reactivation and hepatotoxicity in patients receiving long-term treatment with tumor necrosis factor antagonists. Clin Gastroenterol and Hepatol 2018;16:1964–1973.
- 4
Hwang J and Lok A. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014; 11(4): 209–219.
- 5
Rohit Loomba and T. Jake Liang. Hepatitis B reactivation associated with immune suppressive therapy and biological modifiers: current concepts, management strategies and future therapies. Gastroenterology 2017; 152:1297-1309
- 6
Grace Lai-Hung Wong et al. Impact of dose and duration of corticosteroid on the risk of hepatitis flare in patients with chronic hepatitis B. Liver Int. 2019; 39(2): 271-279
According to international guidelines (AASLD, EASL, and APASL), the decision to treat should be based on clinical characteristics (e.g., extrahepatic manifestations or signs of advanced disease), viral load (HBV DNA), ALT levels, HBeAg status (positive or negative), and intensity of liver damage (inflammation and/or fibrosis).
The indication for treatment addressed here is based on the identification of immunoactive disease (exception for severe acute hepatitis, reactivation, extrahepatic manifestations, cirrhosis, immunotolerant patients, and transplant candidates).
Immunoactive infection, HBeAg positive or negative, is characterized by elevated viral loads (HBV DNA) [>2000–20,000 IU/mL], altered ALT levels [> 1−2x upper limit of normal (ULN)], and degree of histological involvement [A2/F2]. In the follow-up of patients with chronic HBV infection, the situations for treatment indication are often not clearly identified. In these cases, additional characterization or longer follow-up is necessary to establish the best time to start therapy which, in the case of HBV, is almost always life-long. Thus, it is important to establish the situations in which treatment should NOT be indicated, in which the indication is CLEAR, and grey situations that need further clarification.
In most cases, treatment is not indicated for immunotolerant patients (with chronic HBeAg-positive infection) and for inactive carriers (chronic HBeAg-negative infection). On the other hand, treatment is clearly indicated for patients in the immune clearance phase (chronic HBeAg-positive hepatitis) and chronic HBeAg-negative hepatitis characterized by elevated ALT (above the ULN) and a viral load > 2000–20,000 IU/mL. However, there are grey situations that need to be better evaluated before indicating treatment. This category includes immunotolerant patients with mild ALT elevations that can be due to other causes. In these cases, assessments should be repeated every 3–6 months before any decision and a biopsy may eventually be indicated for better clarification. This category also includes HBeAg-negative patients with HBV DNA > 2000 or 20,000 IU/mL but ALT < ULN. These cases require additional clarification by liver biopsy (treat if ≥ A2/F2) or elastography (treat if > 9 kPa). Another grey situation exists when HBV DNA is slightly elevated (< 2000 IU/mL) but ALT > ULN. There may be another cause of ALT elevation in these cases. Clarification with indication of a liver biopsy is recommended.
Pegylated interferon can be used in HBeAg-positive patients with treatment indication, which is more effective in carriers of genotype A and in those with higher ALT levels. The best option for all HBeAg-negative patients are nucleot(s)ide analogs. Entecavir and tenofovir alafenamide are the best options for patients with high risk of renal or bone disease.
In HBeAg-positive patients, treatment should be maintained until seroconversion to anti-HBe. If this response is maintained for at least one to two years, treatment can be discontinued. The patient should be monitored every six months to identify eventual recurrence. In HBeAg-negative patients, treatment should be continued until HBsAg becomes negative (anti-HBs positive) and relapse should be monitored after discontinuation. Treatment should not be discontinued in cirrhotic patients.
Recommendations
- 1
Treatment of hepatitis B should only be indicated after 6–12 months of monitoring ALT levels and viral load (level II-2 evidence, recommendation 1).
- 2
Treatment of hepatitis B is recommended for all patients with evidence of HBeAg-positive or -negative immunoactive disease (level I evidence, recommendation 1).
- 3
Immunoactive disease is characterized by ALT > ULN, HBV DNA > 2000–20,000 IU/mL and evidence of histologically significant disease (A2/F2 in liver biopsy or transient elastography > 9 kPa) (level II-2 evidence, recommendation 1).
- 4
Situations with less evident characteristics of immunoactive disease should be clarified before treatment indication: HBV DNA < 2000 IU/mL and elevated ALT, HBV DNA > 2000–20,000 and ALT ≤ ULN. A liver biopsy or noninvasive test for the detection of significant fibrosis is recommended in these cases (level II-3 evidence, recommendation 1).
- 5
Pegylated interferon, entecavir and tenofovir are the drugs indicated for the treatment of hepatitis B. The use of pegylated interferon for 48 weeks is indicated for HBeAg-positive patients, preferentially genotype A carriers. Entecavir should not be indicated for patients with a history of resistance to lamivudine (level II-1 evidence, recommendation 1).
- 6
Entecavir and tenofovir alafenamide are the preferred drugs for patients at risk of or with renal or bone disease (level I evidence, recommendation 1).
- 7
Therapy with nucleot(s)ide analogs can be discontinued in HBeAg-positive patients with seroconversion to anti-HBe and consolidation of this response for 1–2 years (level II-2 evidence, recommendation 2).
- 8
In HBeAg-negative patients, therapy can be discontinued after HBsAg becomes negative and anti-HBs becomes positive (level II-2 evidence, recommendation 1).
References
- 1
Terrault N, Lok AS, McMahon BJ et al. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: 2018 Hepatitis B Guidance. Hepatology, 67(4):1560-1647.
- 2
Tong MJ, Pan CQ, Hann HW, Kowdley KV, Han SH, Min AD, Leduc TS. Dig Dis Sci. 2011;56(11) :3143-3162
- 3
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98.
- 4
European Association for the Study of the L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-398.
The main goal of antiviral therapy in hepatitis B virus (HBV) carriers with advanced liver disease (compensated or decompensated cirrhosis) is to suppress viral replication to HBV DNA levels that are persistently undetectable by a PCR-based sensitive method (sustained virological response, SVR). Achievement of SVR is associated with lower incidence of progression to liver disease and hepatocellular carcinoma (HCC) and increases liver transplant-free survival. However, the risk of developing HCC is not zero and periodical screening should be maintained indefinitely, even after long periods of SVR and even despite improvement in noninvasive hepatic fibrosis test results.
Limited evidence exists of the impact of antiviral therapy on the evolution of patients with compensated cirrhosis and serum HBV DNA levels less than 2000 IU/mL. However, indirect evidence suggests that cirrhotic patients with a viral load less than 2000 IU/mL are at an increased risk of developing HCC. In addition, a beneficial effect of antiviral therapy on the risk of HCC was observed in these patients.
In patients with HBV-related decompensated cirrhosis, antiviral therapy with nucleoside/nucleotide analogs is associated with improvement of liver function and an increase in overall and liver transplant-free survival, in addition to reducing the risk of HCC. Monotherapy with entecavir (dose of 0.5 mg/day in compensated and 1 mg/day in decompensated cirrhosis) or tenofovir disoproxil is the initially recommended therapeutic regimen. Tenofovir alafenamide has not been studied in patients with decompensated cirrhosis but its use may be considered in selected cases in which the use of tenofovir disoproxil poses a high risk and entecavir is not an option. Tenofovir (disoproxil or alafenamide) is the preferential alternative for patients previously exposed to nucleoside analogs. Entecavir or tenofovir alafenamide is indicated in individuals with evidence or risk of developing kidney injury and/or bone disease. In addition to continuous antiviral therapy, all patient with decompensated cirrhosis should be evaluated periodically by the multidisciplinary team involved in liver transplantation.
Recommendations
- 1
Adults with HBV-related compensated cirrhosis, who are HBsAg positive and have detectable HBV DNA, should receive indefinite antiviral therapy regardless of HBeAg status and aminotransferase levels (level I evidence, recommendation 1).
- 2
Adults with HBV-related decompensated cirrhosis, who are HBsAg positive, should receive indefinite antiviral therapy regardless of HBeAg status and aminotransferase and HBV DNA levels (level II-1 evidence, recommendation 1).
References
- 1
Lok AS, McMahon BJ, Brown RS, Jr., Wong JB, Ahmed AT, Farah W, Almasri J, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 2016;63:284-306.
- 2
Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol 2010;53:348-356.
- 3
Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, Choi MS, et al. Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology 2015;62:694-701.
- 4
Ye XG, Su QM. Effects of entecavir and lamivudine for hepatitis B decompensated cirrhosis: meta-analysis. World J Gastroenterol 2013;19:6665-6678.
- 5
Lee SK, Song MJ, Kim SH, Lee BS, Lee TH, Kang YW, Kim SB, et al. Safety and efficacy of tenofovir in chronic hepatitis B-related decompensated cirrhosis. World J Gastroenterol 2017;23:2396-2403.
- 6
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560-1599.
- 7
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-398.
The phase of HBeAg-positive chronic infection, or immunotolerance, is characterized by HBeAg positivity, high viral load (generally > 106 or 107 IU/mL), alanine aminotransferase (ALT) within the upper limit of normal (ULN), and minimal or absent inflammation and fibrosis upon histopathology. The APASL, EASL and AASLD guidelines are generally uniform in terms of this definition. However, the ULN for ALT varies among the different guidelines, which is 40 IU/L in the 2015 APASL and 2017 EASL consensus (independent of sex), and 35 and 25 IU/L in the 2018 AASLD guidelines for men and women, respectively.
In immunotolerant patients, clearance of HBeAg is rare and the risk of fibrosis progression is generally low. On the other hand, the patient may develop hepatocellular carcinoma (HCC) because of the high HBV replication. In addition, significant histopathological alterations can be observed in immunotolerant patients, especially those older than 30–40 years. Thus, assessment of hepatic fibrosis by noninvasive methods or liver biopsy particularly in this group of patients has been suggested.
The RECOMMENDATIONS for monitoring and treating immunotolerant patients are not uniform across the international guidelines. The APASL consensus recommends noninvasive assessment of fibrosis, monitoring every three months, and indication of a liver biopsy if the noninvasive tests provide evidence of significant fibrosis or in the presence of a family history of HCC or cirrhosis. On the other hand, the European guidelines suggest that treatment should be considered in patients older than 30 years, regardless of histology. If the choice is not to treat, follow-up every three to six months is recommended to assess the risk of HCC, reactivation, transmission, and extrahepatic manifestations. Finally, the AASLD consensus does not recommend treatment of immunotolerant patients but testing of ALT every six months to monitor potential transition to the immunoactive phase. Selected immunotolerant patients (older than 40 years, normal ALT, and elevated HBV DNA > 1 million IU/mL) in whom liver histology shows significant necroinflammation should be treated.
The main arguments against treatment of immunotolerant patients are low rates of HBeAg seroconversion, indefinite period of treatment, lower viral suppression rates, possibility of resistance, and lack of evidence suggesting that treatment would modify the clinical course. On the other hand, the main arguments in favor of treatment are fear that elevated viremia may be oncogenic, possibility of achieving marked viral suppression in almost all patients (even if incomplete), and failure to recognize transition to the immunoactive phase. In a systematic literature review (PubMed and Cochrane databases), among 128 initially evaluated publications, only 10 studies evaluated treatment of this patient profile. Five of these studies included adult patients and only one included non-Asian patients. Two were randomized clinical trials that compared different interventions and the quality of the studies was generally low. These studies showed that nucleos(t)ide analog therapy was able to achieve virological control in immunotolerant patients, but the rate of HBeAg seroconversion was low. An uncontrolled retrospective Korean study including 484 patients, 84 of them treated with nucleot(s)ide analogs, demonstrated that therapy was associated with a reduction in the incidence of HCC and liver cirrhosis. However, in addition to its retrospective design, that study included only Asian patients infected with HBV genotype C and there was no clear standardization of the follow-up of patients prior to inclusion.
Recommendations
- 1
After careful discussion with the patient addressing potential risks and benefits, treatment during the immunotolerant phase should be considered in patients older than 30 years, those with a family history of HCC or cirrhosis, and in cases of extrahepatic manifestations of HBV (level III evidence, recommendation 2).
- 2
Immunotolerant patients not submitted to treatment should be monitored every 3–6 months by ALT measurement to evaluate potential transition to the immunoactive phase (level II-2 evidence, recommendation 1).
- 3
Noninvasive monitoring for the assessment of hepatic fibrosis every 12 months should be considered. A liver biopsy should be obtained when the noninvasive methods indicate significant hepatic fibrosis, in the case of doubt regarding the phase of the disease or, alternatively, during the follow-up of patients older than 30 years or with a family history of HCC/cirrhosis (level III evidence, recommendation 2).
References
- 1
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560-1599.
- 2
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-398.
- 3
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98.
- 4
Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, et al. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology 2007;46:395-401.
- 5
Andreani T, Serfaty L, Mohand D, Dernaika S, Wendum D, Chazouilleres O, Poupon R. Chronic hepatitis B virus carriers in the immunotolerant phase of infection: histologic findings and outcome. Clin Gastroenterol Hepatol 2007;5:636-641.
- 6
Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis 2007;11:797-816, viii.
- 7
Wu ZX, Chen FS, Zhou XL, Huang Q, Zhang SA, Wu HC, Cai LR, et al. Tenofovir and telbivudine combination therapy rapidly decreases viral loads in immune-tolerant chronic hepatitis B patients awaiting assisted reproduction: an open-label, randomized, controlled study. Eur J Gastroenterol Hepatol 2019;31:832-835.
- 8
Chan HL, Chan CK, Hui AJ, Chan S, Poordad F, Chang TT, Mathurin P, et al. Effects of tenofovir disoproxil fumarate in hepatitis B and antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology 2014;146:1240-1248.
- 9
Chang Y, Choe WH, Sinn DH, Lee JH, Ahn SH, Lee H, Shim JJ, et al. Nucleos(t)ide analogue treatment for patients with hepatitis B virus (HBV) and antigen-positive chronic HBV genotype C infection: a nationwide, multicenter, retrospective study. J Infect Dis 2017;216:1407-1414.
The concept of persistent viremia has traditionally been defined as detectable HBV DNA after 48 weeks of treatment. This time point was established based on the outcomes of clinical trials using drugs with lower antiviral potency and higher rates of resistance. With the current therapies based on TDF, TAF and ETV, persistent viremia is defined as a plateau in the decline of HBV DNA levels or as failure to achieve undetectable HBV DNA after 96 weeks of treatment.
Although slow, the constant decline of HBV DNA in these patients does not seem to be associated with poorer outcomes or emergence of resistance and modification of the therapeutic regimen is therefore not indicated. Similarly, minimal residual viremia (HBV DNA < 73 IU/mL) in non-cirrhotic patients does not appear to impact the evolution of these patients. However, in patients with decompensated cirrhosis, failure to achieve virological response (defined as HBV DNA < 20 IU/mL) was associated with higher risk of progression to hepatocellular carcinoma.
Little clinical evidence exists on the management of these patients with low viremia, especially in the case of intermediate values (HBV DNA between 73 and 2000 IU/mL). Before considering virological failure, careful assessment of adherence to treatment is essential. Tests for the evaluation of antiviral drug resistance may be of limited efficacy in this scenario because of low viremia. In patients with low viremia using ETV, TDF or TAF, maintenance of therapy regardless of ALT level is recommended. However, studies suggest that adding another drug to the regimen or switching to another analog may increase the rates of viral suppression and ALT normalization.
Failure of antiviral treatment is defined by an increase of > 1 log compared to nadir HBV DNA or HBV DNA > 100 IU/mL in patients with previously undetectable levels. In treatment-adherent patients, this event is usually due to antiviral resistance, especially when antiviral drugs with low genetic barrier such as lamivudine and adefovir are used. Preventing the emergence of antiviral resistance should be promoted by choosing drugs with high barrier to resistance and high antiviral potential as first-line treatment. The combination or sequential use of drugs with low genetic barrier should also be avoided. Absence of a primary response in adherent patients was described almost exclusively with the use of adefovir, a drug that is no longer recommended by the Brazilian National Health System (SUS) protocols.
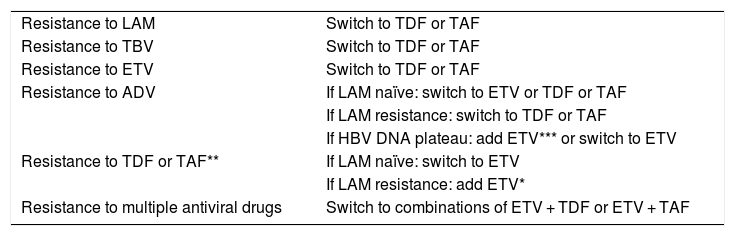
Cases with suspicion of antiviral treatment failure should always be confirmed by evaluating HBV DNA and adherence to treatment. Switching to a regimen using antiviral drugs with high barrier (TDF, ETV, TAF) in monotherapy is recommended for patients on drugs with a low barrier to resistance. Resistance of the virus to antiviral drugs with a high genetic barrier (TDF, ETV, TAF) is uncommon. In this case, therapeutic adjustment should include the most effective antiviral agent that does not show cross-resistance to the initial antiviral drug. The study of antiviral resistance mutations might be useful, especially in patients with previous failures to another antiviral agent. Table 1 shows the EASL recommendations on the management of patients who develop antiviral resistance.
Table 1. Management of patients who develop antiviral resistance.1
| Resistance to LAM | Switch to TDF or TAF |
| Resistance to TBV | Switch to TDF or TAF |
| Resistance to ETV | Switch to TDF or TAF |
| Resistance to ADV | If LAM naïve: switch to ETV or TDF or TAF |
| If LAM resistance: switch to TDF or TAF | |
| If HBV DNA plateau: add ETV*** or switch to ETV | |
| Resistance to TDF or TAF** | If LAM naïve: switch to ETV |
| If LAM resistance: add ETV* | |
| Resistance to multiple antiviral drugs | Switch to combinations of ETV + TDF or ETV + TAF |
1According to J Hepatol 2017;67:370-398.
ETV, entecavir; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; LAM, lamivudine; ADV, adefovir; TBV, telbivudine.
*The long-term safety of this combination is unknown.
**Not observed clinically so far; investigate antiviral resistance mutations in an expert laboratory.
***Especially in patients with ADV resistance mutations (rA181 T/V and/or rN236 T) and high viral load, the response to TDF (TAF) can be protracted
Recommendations
- 1
Adherence to treatment should be evaluated in patients with persistent low viremia or failure of antiviral treatment (level II-1 evidence, recommendation 1).
- 2
In patients with persistent low viremia (HBV DNA < 2000 IU/mL) using ETV, TDF or TAF, the regimen should be maintained in monotherapy regardless of ALT levels (level II-3 evidence, recommendation 3).
- 3
In confirmed cases of antiviral treatment failure, the therapeutic regimen should be readily adjusted taking into consideration data on cross-resistance (Table 1) (level II-2 evidence, recommendation 1).
References
- 1
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560-1599.
- 2
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-398.
- 3
Choi HN, Song JE, Lee HC, Jo HH, Lee CH, Kim BS. Efficacy of prolonged entecavir monotherapy in treatment-naive chronic hepatitis B patients exhibiting a partial virologic response to entecavir. Clin Mol Hepatol 2015;21:24-31.
- 4
Maier M, Liebert UG, Wittekind C, Kaiser T, Berg T, Wiegand J. Clinical relevance of minimal residual viremia during long-term therapy with nucleos(t)ide analogs in patients with chronic hepatitis B. PLoS One 2013;8:e67481.
- 5
Kim SS, Hwang JC, Lim SG, Ahn SJ, Cheong JY, Cho SW. Effect of virological response to entecavir on the development of hepatocellular carcinoma in hepatitis B viral cirrhotic patients: comparison between compensated and decompensated cirrhosis. Am J Gastroenterol 2014;109:1223-1233.
- 6
Ha NB, Ha NB, Trinh HN, Nguyen HA, Nguyen KK, Nguyen MH. Response to higher dose of entecavir 1.0 mg daily in patients with partial response to entecavir 0.5 mg daily. J Clin Gastroenterol 2013;47:461-465.
- 7
Chaung KT, O'Brien C, Ha NB, Nguyen NH, Trinh HN, Nguyen MH. Alternative therapies for chronic hepatitis b patients with partial virological response to standard entecavir monotherapy. J Clin Gastroenterol 2016;50:338-344.
- 8
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH, American Association for the Study of Liver D. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261-283.
Patients who are not candidates for antiviral therapy should be monitored regularly. Immunotolerant HBV carriers (HBeAg-positive chronic infection) can live for a long time with high levels of viral replication and develop little or no liver inflammation. Nevertheless, evidence suggests a strong relationship between high replication rates and progression to hepatocellular carcinoma (HCC). Details on the follow-up and indications of treatment for immunotolerant patients are provided in the section “Treatment of Immunotolerant Patients”. The patients should be followed up at intervals of three to six months for assessing transition to the immunoactive phase. Noninvasive assessment of fibrosis every 12 months should be considered. A liver biopsy in patients initially classified as immunotolerant also plays an important role in follow-up as discussed earlier.
Inactive carriers (HBeAg-negative chronic infection) should have their clinical situation confirmed by quarterly assessment in the first year of follow-up to rule out the presence of HBeAg-negative chronic hepatitis B with fluctuations in inflammatory activity. If available, a quantitative HBsAg result < 1 000 IU/mL will support the diagnosis of an inactive carrier. After the first year, the measurements of aminotransferases and HBV DNA should be repeated every six to 12 months. More frequent reassessments, as well as the use of noninvasive markers of fibrosis and/or liver biopsy, should be considered in the case of increased ALT or HBV DNA level > 2000 IU/mL during follow-up. Annual HBsAg measurement is recommended to detect spontaneous seroconversion.
All chronic HBV carriers should be submitted to HCC screening, which should be personalized according to the clinical characteristics of the patient. However, screening is mandatory in all patients with cirrhosis. HBV patients without cirrhosis but with risk factors for HCC such as ethnicity, age, family history of HCC, coinfection with HCV or HIV and associated non-alcoholic fatty liver disease are also considered a priority for screening.
Ultrasound is the recommended screening method, which should be performed every six months. The combination with alpha-fetoprotein (AFP) as a marker has been a matter of controversy. A recent meta-analysis suggests that, in patients with cirrhosis, adding AFP to the screening strategy increases the sensitivity in detecting HCC. However, data on the performance of AFP in non-cirrhotic AFP carriers are scarce. Thus, its use may be considered in view of regional peculiarities, especially the expertise of the professionals who perform the ultrasound examination.
Patients who achieve HBsAg seroconversion, either spontaneously or induced by treatment, usually show good evolution of liver disease unless another cofactor for liver damage is present. Following confirmation of seroconversion during follow-up for at least one year after its first identification, routine periodic HBV DNA investigation and liver tests can be interrupted. Screening for HCC should be maintained in patients with cirrhosis, with a family history of HCC in a first-degree relative or with a long duration of HBV infection (age > 40 years for men and > 50 years for women in cases of vertical transmission).
Recommendations
- 1
Inactive carriers (HBeAg-negative chronic infection) should be submitted to aminotransferase and HBV DNA assessments every 6–12 months and to annual HBsAg measurement. ALT elevation or HBV DNA > 2000 IU/mL indicates more frequent reassessments, including noninvasive evaluation of fibrosis and/or biopsy (level II-2 evidence, recommendation 1).
- 2
Chronic carriers who are not candidates for therapy should be evaluated regarding HCC risk. Biannual ultrasound screening with/without AFP is recommended for patients with cirrhosis and those with risk factors: Asian ethnicity or Afro-descendants, age (> 40 years for men and > 50 years for women), family history of HCC in a first-degree relative, coinfection with HCV or HIV, and associated nonalcoholic fatty liver disease. Alternatively, all HBsAg-positive patients could be screened for HCC, although this management does not meet cost-effectiveness criteria (level II-2 evidence, recommendation 2).
- 3
Patients who achieve HBsAg seroconversion should have their serology confirmed during follow-up for a minimum of one year. Screening for HCC should be maintained in patients with cirrhosis, with a history of HCC in a first-degree relative or with a long duration of HBV infection (age > 40 years for men and >50 years for women in cases of vertical transmission) (level II-2 evidence, recommendation 1).
References
- 1
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560-1599.
- 2
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-398.
- 3
Kim SS, Hwang JC, Lim SG, Ahn SJ, Cheong JY, Cho SW. Effect of virological response to entecavir on the development of hepatocellular carcinoma in hepatitis B viral cirrhotic patients: comparison between compensated and decompensated cirrhosis. Am J Gastroenterol 2014;109:1223-1233.
- 4
Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65-73.
- 5
Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, Chan HL, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol 2017;32:667-676.
- 6
Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, El-Serag HB, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol 2017;66:355-362.
- 7
Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of metabolic risk factors on risk of hepatocellular carcinoma and liver-related death in men with chronic hepatitis B: A large cohort study. Gastroenterology 2017;153:1006-1017 e1005.
- 8
Huang YT, Yang HI, Liu J, Lee MH, Freeman JR, Chen CJ. Mediation analysis of hepatitis B and C in relation to hepatocellular carcinoma risk. Epidemiology 2016;27:14-20.
- 9
Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology 2013;57:249-257.
- 10
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50.
- 11
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
- 12
Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology 2018;154:1706-1718 e1701.
Patients with hepatitis B can be monoinfected or coinfected. The most common hepatitis B coinfections are:
- A)
Coinfection with HCV
- B)
Coinfection with HIV
- C)
Coinfection with HDV
A) HBV/HCV coinfection
Because they share similar transmission routes, HBV/HCV coinfection is more common in regions endemic for both viruses. The global prevalence of this coinfection ranges from 1% to 15%. Some studies have shown that HCV is usually dominant and HBV can be serologically evident or occult.
The natural course of HBV/HCV coinfection has a poor prognosis and therefore requires adequate treatment. Known for a long time, these two viral infections have been treated with the same medication, i.e., interferon. At that time, there were no reports of reactivation of HBV when HCV was eradicated. More recently, with the advent of direct-acting antiviral (DAA) treatment, cases of HBV reactivation, including progression to fulminant hepatitis, were reported during or after DAA treatment of patients coinfected with HBV/HCV who did not receive HBV suppression. The FDA identified 24 cases of HBV reactivation in coinfected patients treated with DAAs over a period of 31 months (2013–2016). The baseline features of these patients were heterogenous, including individuals with inactive, occult, and past HBV infection. Three patients developed fulminant hepatitis as a result of HBV reactivation and two died, one had past HBV infection and the other had undergone liver transplantation. In subsequent cohorts of patients treated with DAAs, HBV reactivation was common among those with detectable HBsAg and less frequent among those with anti-HBc alone.
Recommendations
- 1
Before starting therapy with DAAs, patients infected with HCV should be tested for HBV coinfection using HBsAg and for past infection using anti-HBs and anti-HBc (level II-2 evidence, recommendation 1).
- 2
HBsAg-positive patients who do not meet the criteria for HBV treatment should receive antiviral prophylaxis for HBV for at least 12 weeks after hepatitis C treatment (level II-2 evidence, recommendation 2).
- 3
In HBsAg-negative and anti-HBc-positive patients, serum ALT levels should be monitored monthly. If ALT is elevated, the patient should be retested for HBsAg and HBV DNA (level III evidence, recommendation 2).
- 4
If HBsAg and/or HBV DNA become positive after the use of DAAs, HBV treatment should be initiated (level II-2 evidence, recommendation 1).
- 5
The antiviral drugs of choice for the treatment of HBV in HBV/HCV coinfection are entecavir, tenofovir disoproxil, and tenofovir alafenamide (level I evidence, recommendation 1).
B) HBV/HIV coinfection
Data on the treatment of HBV/HIV coinfected patients with interferon are limited and not very encouraging. In addition, studies in which treatment was intensified by combining pegylated interferon with adefovir or tenofovir (TDF) for one year found no increase in HBV seroconversion rates.
Regarding therapy with nucleos(t)ides, adefovir should not be used because it has no activity against HIV. Lamivudine, emtricitabine and tenofovir are analogs with activity against both HBV and HIV. In view of the rapid development of resistance when HBV is not completely suppressed, monotherapy with lamivudine or emtricitabine should not be considered. Thus, the treatment of choice for HBV is tenofovir (TDF). This drug is combined with emtricitabine or lamivudine in most current antiretroviral regimens. Tenofovir alafenamide was approved for the treatment of HIV in combination with emtricitabine with or without other HIV drugs and is preferable to TDF because of its better safety profile.
Entecavir may be a therapeutic alternative for hepatitis B; however, the drug only reduces HIV RNA levels and its use may result in the selection of the M184V mutation. It should therefore only be used in HBV/HIV-coinfected patients with complete HIV suppression. The addition of pegylated interferon to antiretrovirals that are active against HBV did not increase the clearance rates of HBeAg or HBsAg despite a faster decline in antigen levels during treatment.
Recommendations
- 1
All HBV/HIV-coinfected patients should receive antiviral therapy that includes two medications active against HBV, specifically tenofovir combined with lamivudine or emtricitabine (level I evidence, recommendation 1).
- 2
Tenofovir alafenamide is preferable to tenofovir disoproxil always when there is concern regarding the safety profile, particularly renal and bone (level II-1 evidence, recommendation 1).
- 3
When the antiretroviral treatment is altered, drugs effective against HBV should not be discontinued without replacement by another medication active against HBV (level II-2 evidence, recommendation 1).
References
- 1
AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org [accessed 15 September 2019]
- 2
Terrault NA, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560-1599
- 3
Singh KP, et al. HIV-hepatitis B virus co-infection: epidemiology, pathogenesis and treatment. AIDS. 2017; 31(15):2035-2052.
- 4
EASL Recommendations on treatment of hepatitis C. Journal of Hepatology. 2018
- 5
Londoño MC, Lens S, Mariño Z, Bonacci M, Ariza X, Broquetas T, Pla A, et al. Hepatitis B reactivation in patients with chronic hepatitis C undergoing anti-viral therapy with an interferon-free regimen. Aliment Pharmacol Ther. 2017;45:1156-1161.
- 6
Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, Karlberg J, Lau G. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: A systematic review and meta-analysis. Hepatology. 2017;66(1):13-26.
C) HBV/HDV coinfection
Coinfection of HBV with hepatitis delta virus (HDV) occurs in about 5% of HBsAg carriers. The global distribution of HBV/HDV coinfection is irregular and focal. In Brazil, this coinfection predominates in western Amazon region, with sparse foci in the southern and southeastern regions. Testing for HDV infection should potentially be considered in all HBsAg-positive patients. However, there are no studies that evaluated the cost-effectiveness of this universal testing. Thus, diagnostic testing is recommended for individuals living in HDV-endemic areas or those traveling through these areas, as well as other risk groups such as intravenous drug users.
Hepatitis delta is extremely pathogenic and frequently progresses to cirrhosis, with the observation of high rates of hepatocellular carcinoma and mortality when compared to HBV-monoinfected patients.
Liver elastography methods have not yet been validated for hepatitis delta and therefore cannot be recommended. On an individual basis and in an adequate clinical context, these methods might be useful to identify the extremes of fibrosis, advanced or minimal. In cases without clinical evidence of cirrhosis, a more accurate diagnosis of liver involvement should be made by biopsy. Considering the high pathogenicity of HDV, treatment can be indicated on an individual basis for viremic patients with biochemical evidence of liver aggression in the absence of a biopsy.
The recommended treatment is pegylated interferon for 48 weeks. Predictors of treatment response have not been adequately validated for HDV infection. The available data permit to recommend, with a low level of evidence, the assessment of viral load at six months and at the end of treatment. Patients who are negative for HDV RNA at six months and at the end of treatment have the best chances of SVR. Patients who exhibit a decline in viral load at six months without achieving undetectable viremia may benefit from the prolongation of treatment to 72 weeks. Considering the possibility of late recurrence, long-term laboratory clinical follow-up should be individualized even for patients who achieved a virological response after six months of treatment.
Recommendations
- 1
Investigation of HDV infection is recommended in HBsAg-positive patients who live in endemic areas or have a history of travel to these areas (level II-2 evidence, recommendation 1); patients with risk factors for parenteral exposure, multiple sex partners and a history of sexually transmitted diseases, men who have sex with men, patients coinfected with HIV or HCV, patients with acute or particularly severe chronic conditions or evidence of hepatic aggression with low HBV load (level II-3 evidence, recommendation 2).
- 2
Infection should be investigated at diagnosis and during follow-up, whenever there is evidence of liver disease aggravation (level II-2 evidence, recommendation 1).
- 3
A liver biopsy is recommended for viremic patients without clinical-laboratory evidence of chronic liver disease (level II-2 evidence, recommendation 1).
- 4
Viremic patients (detectable HDV RNA) with evidence of active liver disease should be treated (level II-2 evidence, recommendation 1).
- 5
The currently available treatment is pegylated interferon for 48 weeks (level I evidence, recommendation 1).
- 6
The duration of treatment can be extended on an individual basis to 72 weeks if the patient shows a slow virological response and good tolerance to treatment (level II-2 evidence, recommendation 1).
- 7
Patients with evidence of significant HBV replication (HBV DNA > 2000 IU/mL) should be treated with pegylated interferon combined with a nucleos(t)ide analog (level II-2 evidence, recommendation 2).
- 8
Nucleos(t)ide analog monotherapy can be used in patients with detectable HBV DNA who cannot be treated with pegylated interferon, such as those with decompensated liver cirrhosis (level II-2 evidence, recommendation 1).
References
- 1
Hughes AS, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet 2011;378(9785):73-85.
- 2
Koh C, Da BL, Glenn JS. HBV/HDV coinfection: a challenge for therapeutics. Clin Liver Dis. 2019; 23(3):557-72.
- 3
Heidrich B, Manns M, Wedemeyer H. Treatment options for hepatitis delta infection. Curr Infect Dis Rep 2013; 15(1):31-8.
- 4
Keskin O, Wedemeyer H, Tuzun A, Zachou K, Deda X, Dalekos GN et al. Association between level of hepatitis D virus RNA at week 24 of pegylated interferon therapy and outcome. Clin Gastroenterol Hepatol. 2015; 13(13): 2342-49.
Antiviral therapy usually prevents the need for liver transplantation in patients with hepatitis B, even in advanced stage. Nevertheless, decompensated cirrhosis remains an important indication for this procedure. Hepatocellular carcinoma detected in the cirrhotic liver is another common indication.
After transplantation, prophylactic treatment should always be implemented in these patients in order to reduce the risk of HBV recurrence in the graft, ensuring better survival rates of the patient and of the graft. This strategy reduces the rate of reinfection in the transplanted organ to less than 5–10%. Antiviral therapy with potent nucleoside/ nucleotide analogs (NUC) such as entecavir, tenofovir (TDF) or tenofovir alafenamide (TAF) prevents the recurrence of infection after transplantation but the drugs should be maintained indefinitely.
Most liver transplant centers in the world use anti-HBV immunoglobulin (HBIg) during the early post-transplant period, with variable doses and durations depending on each center. In patients at high risk of HBV recurrence during the post-transplant period (e.g., patients with positive HBV DNA at the time of transplantation, HBeAg-positive patients, patients with hepatocellular carcinoma, and patients coinfected with HIV or HDV), combination therapy, i.e., HBIg combined with a potent NUC, should be initiated and continued indefinitely.
With respect to HBIg, most transplant centers recommend personalized prophylaxis of HBV recurrence in which HBIg is first administered intravenously for 5–7 days. Subsequently, this formulation is replaced with a more convenient dosage administered subcutaneously or intramuscularly and continued indefinitely. HBIg is also indicated for application during the anhepatic phase of transplant surgery to neutralize circulating viral particles during placement of the new organ. The antiviral drugs used in combination should be highly potent, with a minimum risk of developing resistance over the years. Using these strategies, the percentage of HBV prevention in these patients is about 97%. In patients who continue to receive combination therapy with potent NUC and HBIg, anti-HBs levels should be monitored and the aim is to achieve serum levels ≥ 50–100 IU/mL.
Adverse effects of the drugs, particularly those related to TDF, have been reported, including acute kidney damage and bone alterations. Monitoring of these effects is therefore necessary, mainly because the combination of antiviral drugs with calcineurin inhibitors used to prevent organ rejection can potentiate the occurrence of kidney failure. The use of TAF instead of TDF would be indicated in these cases.
Recommendations
- 1
Patients with hepatitis B who are candidates for a liver transplant should be treated with oral nucleos(t)ides in an attempt to achieve undetectable HBV DNA at the time of transplantation (level II-1 evidence, recommendation 1).
- 2
The combination of a potent NUC (ETV/TDF/TAF) with hyperimmune globulin (HBIg) can have a synergistic effect aimed at achieving anti-HBs levels ≥ IU/L (level II-2 evidence, recommendation 1).
- 3
Combination therapy (NUC/HBIg) should be given indefinitely to all patients at high risk of HBV recurrence, such as i) patients who are HBV DNA-positive at the time of transplantation; ii) HBeAg-positive patients; iii) patients with hepatocellular carcinoma; iv) HIV- or HDV-coinfected patients (level II-2 evidence, recommendation 1).
- 4
The various commercial preparations of HBIg (intravenous, subcutaneous or intramuscular) should never be used in monotherapy after transplantation. The daily doses range from 1000 to 5000 IU when administered intravenously. In the case of continued use for an indefinite period of time, subcutaneous or intramuscular administration is preferred, with personalized dosing (level II-3 evidence, recommendation 2).
- 5
In selected patients who are HBV DNA negative at the time of transplantation, intravenous HBIg during the anhepatic phase and for more 5–7 days should be considered and can later be discontinued, as long as prophylaxis with a potent NUC is continued indefinitely (level II-2 evidence, recommendation 2).
- 6
HBsAg-negative patients receiving anti-HBc-positive grafts (past or occult infection) are at risk of recurrence and should receive continuous antiviral prophylaxis with a potent NUC (level II-2 evidence, recommendation 1).
References
- 1
Coffin, C. S et al. Virologic and clinical outcomes of hepatitis B virus infection in HIV-HBV coinfected transplant recipients. Am J. Transplant 2010: 10: 1268 -1275.
- 2
De Simone et al. Early introduction of subcutaneous Hepatitis B immunoglobulin following liver transplantation: a Prospective, multicenter Study. Transplantation 2016;100:1507 -1512.
- 3
EASL Guideline Hepatitis B 2017. AALSD Guideline Hepatitis B 2018.
- 4
Beckebaum et al. Recurrence of hepatitis B infection in liver transplant patients receiving long term hepatitis B immunoglobulin prophylaxis. Ann Transplant 2018; 23: 789-801.
- 5
Cholongitas et al. Hepatitis B immunoglobulin and/or NUC for prophylaxis against hepatitis B recurrence after liver transplantation: a systematic review. Liver Transpl 2011: 17 (10): 1176 -90.
The peculiarity of hepatitis B in childhood are the high rates of chronicity after maternal-fetal transmission. If the child contracts the virus by maternal transmission, evolution to chronicity occurs in 90% of cases, while at 1–5 years the rate of chronicity is about 30%. In contrast, the rates of chronicity among adults ranges from 2% to 5%.
Maternal-fetal contamination can currently occur during different phases of pregnancy, which range from infected semen or oocytes, amniotic fluid, placenta or contaminated monocytes to trauma during labor. Thus, it is not sufficient to try to prevent hepatitis B only after delivery but pregnant women require specific precautions in order to avoid contamination of the embryo and fetus whenever the mother is a HBV carrier.
The risk factors for vertical transmission include HBeAg-positive status, HBV DNA levels > 106 IU/mL, and coinfection with HIV. Other factors such as age < 25 years and Asian ethnicity are also considered risk factors. Considering these factors, prophylactic treatment of hepatitis B in the last trimester of gestation is a valid option to prevent maternal-fetal transmission.
In Brazil and all over the world, the recommendation is to vaccinate all newborns independent of the mother’s HBV status with three doses of hepatitis B vaccine, with the first dose being administered within 48 h after birth still in the maternity unit. The other two doses will be given at two and six months of age together with pentavalent vaccine.
In children born to HBsAg-positive mothers, one to two months after completing vaccination schedule, HBsAg and anti-HBs should be determined. HBsAg negative and anti-HBs > 10 UI/mL indicate immunity acquired from vaccination; children with HBsAg negative and anti-HBs negative should be revaccinated; and those with HBsAg positive should be referred to specialist since they present HBV infection due to vaccination failure.
All newborns whose mother is a carrier of HBV (HBeAg positive or negative) should receive the first hepatitis B vaccine dose and hyperimmune gammaglobulin (HBIg) intramuscularly at different and contralateral sites within the first 12 h of life. The children should subsequently receive three additional doses at 2, 4 and 6 months together with the pentavalent vaccine. These children born to HBV carriers should be tested for HBsAg and anti-HBs one to two months after completion of the vaccination regimen. The titers of the latter should be > 10 IU/mL. If this is not the case, HBV DNA testing is necessary to rule out occult HBV infection. If HBV DNA is absent, the child should receive three additional vaccine doses at regular intervals, followed by monitoring. As in adults, a third vaccination schedule is not recommended if there is no response.
Regardless of the route of transmission, children carrying HBV should be monitored every six months by measuring ALT, HBsAg and HBeAg. For immunotolerant children, i.e., with chronic HBV infection but without chronic hepatitis B, the management is similar to that recommended for adults and they should not be submitted to antiviral treatment during this phase. The treatment indications for children are similar to those for adults but fewer drugs are available (pegylated interferon after three years of age, entecavir after two years, and tenofovir (TDF) after 12 years)
Recommendations
- 1
All pregnant women should be tested for HBsAg in the first trimester (level II-2 evidence, recommendation 1).
- 2
Hepatitis B vaccination of susceptible pregnant women at the beginning of pregnancy (level II-2 evidence, recommendation 1).
- 3
In the third trimester of gestation, HBsAg should be requested together with HBeAg and anti-HBe (level II-2 evidence, recommendation 1).
- 4
The HBV load guides the therapeutic management in HBsAg-positive pregnant women and should be obtained in the third trimester, whenever possible (level II-2 evidence, recommendation 2).
- 5
All HBsAg-positive/HBeAg-positive pregnant women should receive prophylactic treatment in the third trimester of gestation (level II-2 evidence, recommendation 2).
- 6
In HBsAg-positive and anti-HBe-positive pregnant women, viral load should be determined in the third trimester and these women should be treated if HBV DNA > 200,000 IU/mL (level II-3 evidence, recommendation 2).
- 7
Pregnant women should be treated with TDF at habitual doses, preferentially up to the third month after delivery (level II-2 evidence, recommendation 1).
- 8
During breast-feeding, to avoid exposure of the child, the drug should be taken at least 4 h before feeding (level II-3 evidence, recommendation 2).
- 9
The criteria for treating children with HBV infection are: HBsAg positive, HBV DNA > 2000 IU/mL, and ALT > 1.5x ULN. (level II-2 evidence, recommendation 1).
- 10
A liver biopsy is indicated in doubtful cases to evaluate the hypothesis of chronic hepatitis: moderate/severe inflammatory activity with or without fibrosis (level II-2 evidence, recommendation 2).
- 11
The following drugs can be given to children in Brazil: pegylated interferon 2a at a dose of 180 microg/1.73 m2 1x/week for 6–12 months for children ≥ 3 years; entecavir at a dose of 0.15 mg/kg/day orally for children > 2 years; TDF at a dose of 300 mg/day orally for children > 12 years (level II-1 evidence, recommendation 1).
References
- 1
Indolfi G, Easterbrook P, Dusheiko G et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol 2019; 4: 466–76
- 2
WHO. Global hepatitis report, 2017. Geneva: World Health Organization, 2017.
- 3
Bortolotti F, Guido M, Bartolacci S, et al. Chronic hepatitis B in children after e antigen seroclearance: final report of a 29-year longitudinal study. Hepatology 2006; 43: 556–62.
- 4
Sokal EM, Paganelli M, Wirth S, et al. Management of chronic hepatitis B in childhood: ESPGHAN clinical practice guidelines: consensus of an expert panel on behalf of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Hepatol 2013; 59: 814–29.
- 5
Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018; 67: 1560–99.
The authors declare no conflicts of interest.