Effective and long-term combined antiretroviral therapy (cART) has decreased morbidity and mortality in HIV-infected individuals. Despite treatment advances, HIV-infected children continue to develop noninfectious conditions, including liver fibrosis.
MethodsCross-sectional study designed to identify liver fibrosis in HIV-infected adolescents and young adults, in an outpatients clinic of Pediatric Infectious Diseases Division at Escola Paulista de Medicina/Universidade Federal de São Paulo (UNIFESP), diagnosed by noninvasive methods (liver elastography–FibroScan®, APRI and FIB4). Variables examined included demographics, clinical, laboratories, HIV treatment. All participants underwent FibroScan® to measure liver parenchyma elasticity. Values equal to above 7.0 kPa were interpreted as the presence of significant liver fibrosis. Two different biomarkers of liver fibrosis were employed: the AST-to-Platelet Ratio Index (APRI) and the Fibrosis-4 score (FIB-4). APRI values above 1.5 have been considered as levels of clinically significant liver fibrosis and FIB-4 values above 3.25 suggested the presence of advanced fibrosis.
ResultsBetween August 2014 and March 2017, the study enrolled 97 patients, age 10–27 years old, fourteen of 97 subjects (14.4%) presented liver stiffness (≥7 kPa) detected by the liver elastography. No patient had APRI> 1.5. No patient had FIB4 value > 3.25. The only isolated laboratory parameter that could be significantly associated with high liver stiffness was thrombocytopenia (p = 0.022, Fisher's exact test).
ConclusionLiver stiffness was identified in 14.4% (14/97) of this cohort by liver elastography. Liver disease in HIV-infected adolescents and young adults manifests itself silently, so should be routinely investigated.
The introduction of effective Combined Antiretroviral Therapy (cART) significantly reduced morbidity and mortality of human immunodeficiency virus (HIV)-infected patients.1 However non-infectious complications, including liver disease, have become significant causes of long-term morbidity and mortality in HIV-infected patients.2
Liver disease is serious and potentially fatal in HIV coinfected and in HIV-monoinfected patients and can occur in a variety of ways. There are direct and indirect mechanisms that may contribute to the progression of liver disease. HIV causes direct cytopathic effects in the liver cells.3 Indirectly, long-term antiretroviral use like nucleoside analog reverse transcriptase inhibitors, long-term inflammation and metabolic complications may also contribute to the pathogenesis of liver disease.4
Liver disease can be silent and symptoms typically arise in advanced stages, as described in some adolescents exposed long-term to didanosine (ddI).5–7 Liver fibrosis, one of the hepatic complications of HIV infection, was previously associated with the following factors in adults: splenomegaly, prolonged use of didanosine (ddI), thrombocytopenia, elevation of serum aminotransferase and alkaline phosphatase.8
An unexplained hepatic fibrosis with noncirrhotic portal hypertension in an adolescent without viral hepatitis co-infection was identified in our cohort of perinatally HIV-infected patients.9 This event triggered the prospective investigation of liver disease in our patients. The aim of this study was to assess liver fibrosis by non-invasive methods in a cohort of perinatally HIV-infected adolescents and young adults. Detecting potential, subclinical liver disease in this population is important not only to prevent complications, but also to plan therapeutic antiretroviral management.
MethodsThis was a cross-sectional study designed to assess liver diseases in a cohort of perinatally HIV-infected adolescents and young adults using noninvasive methods. Between August 2014 and March 2017, all 115 patients followed at the Pediatric Infectious Diseases Division at Escola Paulista de Medicina/Universidade Federal de São Paulo (UNIFESP) were invited to participate in the study. Only perinatally HIV-infected patients were included and those with hepatitis B or C, alcohol consumption, drug-abuse, use of estrogens, hepatotoxic drugs and other viral hepatitis were excluded. Nine subjects declined, one was excluded due to hepatitis C co-infection and eight were not perinatally HIV-infected. Ninety-seven patients were enrolled in this study.
Demographic and laboratorial parametersVariables examined included age, sex, race, pediatric AIDS classification,10 weight, height, body mass index (BMI),11 date of HIV infection diagnosis, presence of hepatosplenomegaly, exposure time to cART, exposure time to ddI, HIV viral load, current and nadir CD4+ T cell count, current CD8+T cell count, current CD4/CD8 ratio, and laboratory variables (platelets count, serum alanine aminotransferase–ALT, aspartate aminotransferase–AST, gamma-glutamyltransferase–GGT, Alkaline Phosphatase–AP, glycemia, insulin, triglycerides, and total cholesterol). Clinical and laboratory parameters were collected from the patients’ medical records. Laboratorial parameters, when not available at enrollment, results of samples collected within three months were used.
Transient hepatic elastography (THE)THE examination was performed by an experienced investigator in all subjects at study entry at the Hepatology Branch of the Division of Gastroenterology at Escola Paulista de Medicina/UNIFESP, using a FibroScan 502 equipment (EchoSens, Paris, France). Measurements were performed using the standard technique, as previously described in some studies.13–16 Only patients with at least 10 valid measurements in the same THE procedure, with an interquartile range of less than 30% of the median stiffness, and with at least 60% success rate were included in the final analysis.12
According to previous studies, THE values equal or above 7.0 kPa were interpreted as the presence of significant liver stiffness, which corresponds to septal fibrosis, and values above 12.5 kPa indicated the presence of cirrhosis.12 The diagnostic performance of THE was confirmed by several meta-analyses that confirm its excellent diagnostic accuracy for cirrhosis of (>90%).13–16
Non-invasive serum biomarkersTwo different biomarkers of liver fibrosis were employed: the AST-to-Platelet Ratio Index (APRI) and the Fibrosis-4 score (FIB-4). The APRI was calculated according to the formula: [(current AST[U/L] ⁄ Normal AST [U/L) / (Platelet count x 109 ⁄ L)] x 100.17 The FIB-4 was calculated using the formula: age (years) x AST [U/L] / (platelets [109/L] x (ALT [U/L])1/2).18
Patients with APRI values above 1.5 have been considered with clinically significant liver fibrosis (at least septal fibrosis)17 and FIB-4 values above 3.25 suggested the presence of advanced fibrosis.19
Statistical analysisInitially the data were analyzed descriptively. Absolute and relative frequencies were used for the categorical variables and for the numerical variables, summary-measures (mean, median, minimum, maximum, and standard deviation).
The associations between two categorical variables were verified using the Chi-Square test, or alternatively Fisher's exact test in cases of small samples. The comparison of the medians between two groups was performed using the non-parametric Mann–Whitney test due to violation of the assumption of normal distribution in the variables.
EthicsThis study was approved by the Institutional Review Board (IRB) of UNIFESP. Written informed consent was obtained from the parents or legal guardian and patients older than 18 years old. Consent was obtained from adolescents younger than 18 years old.
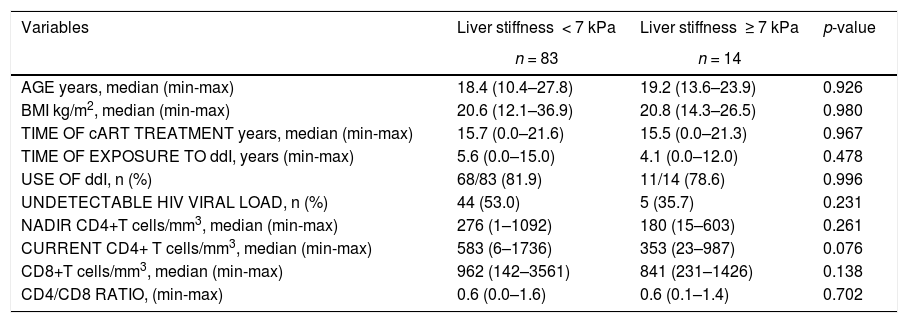
ResultsMost of the patients were female 60/97 (61.9%), white 64/97 (66.0%), aged between 10 and 27 years old with median age of 18.6 years and 85/97 (87.6%) were clinically categorized as B and C and 83/97 (85.6%) immunologically as 2 and 3 of the pediatric AIDS classification CDC1994. Half of them had undetectable HIV viral load at enrollment in the study. In spite of the median current CD4+ T cell count within normal levels, these patients had very low CD4+ T cells nadir. The majority of subjects were on cART for many years (Table 1). Only 2.1% (2/97) were not on cART at inclusion in this study. 52.6% (51/97) were exposed to ddI for five years or more.
Main demographic, virologic, immunological and therapeutic characteristics of patients with and without elevated liver stiffness (LS) assessed by transient hepatic elastography (THE).
p: Fisher's exact test or Chi-Square or Mann–Whitney test.
BMI: Body mass index.
Fourteen of 97 subjects (14.4%) presented liver stiffness ≥ 7 kPa by THE and median elastography was 5.4 kPa (range: 3.0–16.9 kPa). Out of 14 patients with elastography result > 7 kPa, five had elastography result above 11 kPa.
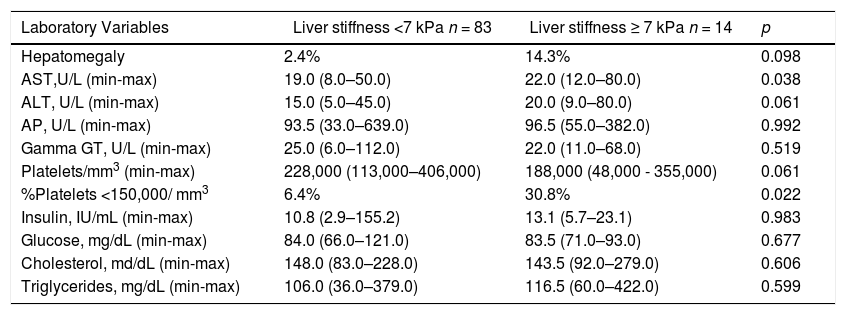
According to clinical evaluation, 80.4% (78/97) of the patients had normal body mass index (BMI), 7.2% (7/97) were underweight, 8.2% (8/97) were overweight, 2.1% (2/97) were obese and 2.1% (2/97) had short stature. Two out of eight overweight patients had THE measurement above 7 kPa. None of the obese patients had liver stiffness above 7 kPa. Only one obese patient met the criteria for metabolic syndrome. One patient had splenomegaly and four patients had hepatomegaly (Table 2). Elevated AST, ALT, AP, GGT were found in 2/97 (2.1%), 2/96 (2.1%), 2/95 (2.1%), 6/90 (6.7%) patients, respectively. Nine of 91 evaluated patients (9.9%) had platelets count < 150.000/mm3. Four out of nine patients with thrombocytopenia (platelet count less than 150,000/mm3) had presumed significant liver fibrosis (liver stiffness above 7 kPa).
. Main Laboratory characteristics in patients with and without elevated liver stiffness (LS) assessed by transient hepatic elastography.
p - Mann–Whitney test.
AST- Aspartate aminotransferase.
ALT-Alanine aminotransferase.
AP-Alkaline Phosphatase.
GGT-Gamma-glutamyltransferas.
APRI index was calculated for all subjects with a median value of 0.2 (range: 0.04–1.18); no patient had APRI>1.5. FIB4 index was also calculated for all subjects with a median value of 0.4 (range: 0.1–1.3); no patient had values over 3.25.
The only isolated parameter that could be significantly associated with significant liver fibrosis was platelet count under 150,000 platelets/mm3 (p = 0.022, Fisher's exact test) (Table 2). Likewise, AST levels were higher in those patients, when compared to patients who had normal THE (p = 0.038, Mann–Whitney test) (Table 2) and ALT levels tended to be higher too, although this was not statistically significant (p = 0.061, Mann–Whitney test).
CD4+ T lymphocyte counts had a tendency to be higher in patients with normal THE, when compared to those with altered values (p = 0.076, Mann–Whitney test) (Table 1).
There was no association between time on ddI and presence of significant liver fibrosis diagnosed by THE. The time on ddI was similar in the those with and without liver stifness.
DiscussionAs a result of increasing survival of HIV-infected patients, noninfectious complications became frequent and significant. Progressive liver injury is a concern in subjects exposed to long-term antiretroviral drugs and the cytopathic effect of HIV infection could have silent evolution.5–7
Considering the progression of the injury, early liver disease diagnosis is very important. Non-invasive methods for liver disease investigation in HIV-infected children and adolescents, predominantly the APRI and FIB4 indexes, have been used by some investigators.20–22 APRI, FIB-4 and THE have higher diagnostic accuracy for ruling out, rather than for ruling in the presence of significant/advanced fibrosis, with negative predictive values above 90%, particularly to exclude advanced liver fibrosis (nodular fibrosis or cirrhosis).17,18 As compared to APRI and FIB-4, THE exhibits similar accuracy to detect septal liver fibrosis and higher accuracy to identify cirrhosis.23 Since APRI and FIB-4 are cheaper and more widely available, they are ideal screening tests, mainly in non-specialized settings.
In addition to these methods, we used THE which demonstrated a prevalence of significant liver fibrosis of 14.4% (14/97). One study, in HIV mono-infected adults without use of ddI-containing cART, showed 9.9% (10/101) of significant liver fibrosis (THE≥7.2).24 Another study with 59 HIV mono-infected adults with elevated aminotransferase level for more than six months showed a higher proportion of this condition (42%) demonstrating that THE≥7.1 had a high sensitivity and specificity for detecting moderate fibrosis.25
By contrast, a study evaluating adult hepatitis C monoinfected and HIV coinfected patients using different cutoffs, THE >6.8 kPa, APRI>0.6, FIB-4>1.4 showed low sensitivity for all noninvasive methods (70% THE, 54% APRI, 59% FIB-4).26
In our investigation no patient had APRI index>1.5, or FIB-4 > 3.25. Therefore, in this cohort, 14 cases with significant/advanced fibrosis would have been missed had these methods been used not in association with THE. These findings emphasize the higher accuracy of THE, as compared to APRI and FIB-4.
Study in HIV-infected children and adolescents also found high prevalence of liver fibrosis using APRI and FIB-4 indexes. Liver disease was diagnosed in 20/79 (25%) of the patients, including 13/71 (18%) participants without coinfection and 7/8 (88%) with hepatitis B or C coinfection.20
This study showed that laboratory and clinical factors associated with significant liver fibrosis diagnosed by THE were higher AST and percentage of patients with thrombocytopenia. Interestingly, those same variables are combined to form the APRI index, which has exhibited a low sensitivity in our cohort, when fibrosis stage was estimated through THE. Unknown factors specifically related to HIV infection could have been implicated in this poor diagnostic performance of APRI. Another study in HIV adults found that age and BMI had a positive association with liver stiffness, while CD4/CD8 ratio was negatively associated.24 Age, BMI and CD4/CD8 ratio were not associated with altered transient elastography in the present study (Table 1). It is possible that early institution of HAART seen in the present cohort (perinatally HIV-infected adolescents and young adults) could have abrogated the negative impact of these variables. Nevertheless, we observed a trend of lower current CD4+ T lymphocyte counts in patients with abnormal THE (Table 1).
Prolonged use of ddI is strongly associated with liver nodular regenerative hyperplasia (NRH)27 and complications associated with non-cirrhotic portal hypertension.28 Use of ddI for over 11 years, as well as the combined use of ddI and stavudine for more than four years, has been characterized as risk factors for the development of non-cirrhotic portal hypertension in HIV-infected patients.29 No association between time on ddI and significant liver fibrosis diagnosed by THE was observed in our study. In contrast, another research found a low prevalence (2%) of previously undiagnosed ddI-associated NRH using a screening strategy that combined THE, serum aminotransferase and platelet measurements followed by an ultrasound.30
Imaging methodologies under study, as ultrasound, magnetic resonance and magnetic resonance elastography, are promising new approaches that provide simultaneous diagnosis, staging, and prognostic information for liver disease.31
The diagnosis of hepatic fibrosis using noninvasive methods is well established and validated for hepatitis C, both in Brazil32 and in other countries.33 However, for HIV infection, noninvasive methods to identify hepatic fibrosis do not yet have a well-defined standardization. Due to the invasiveness of the procedure, we could not compare elastography with hepatic biopsy, which is the gold standard for assessing liver fibrosis. These factors, associated with the absence of a control group, might be limitations of this study.
New studies are needed to clarify the best procedures to optimize the diagnosis of hepatic fibrosis, especially in the young HIV-infected population in order to avoid late diagnosis and its complications. Based on the available data in the literature, and analyzing our findings, we conclude that THE can be considered a reliable method for detecting liver fibrosis. In non-specialized settings, the presence of elevated AST levels and low platelet count should incite the need for THE evaluation.
ConclusionLiver stiffness was identified in 14.4% (14/97) of this cohort by liver elastography. Liver disease in HIV-infected adolescents and young adults manifests itself silently, so should be routinely investigated.