São Paulo city has been one of the regions most affected by the COVID-19 pandemic in Brazil. Frequent asymptomatic and oligosymptomatic infections and poor access to diagnostic tests make serosurveys crucial to monitor the magnitude of the epidemic and to inform public health policies, such as vaccination plans.
ObjectivesTo estimate, early in the epidemic, the seroprevalence of antibodies to SARS-CoV-2 in adults living in the six most affected districts in São Paulo city, and to assess potential associated risk factors.
MethodsThis was a cross-sectional population-based survey of 1,152 households randomly selected from 72 census tracts. During the period May 4–12, 2020, 463 participants completed a questionnaire on sociodemographic characteristics and history of symptoms in the past two weeks, and provided a blood sample. Prevalence of SARS-CoV-2 antibodies was the outcome of interest and was estimated based on results of two immunoassays, Maglumi SARS-CoV-2 chemiluminescence assay Immunoglobulin (Ig) M (IgM) and IgG, and Roche electrochemiluminescence assay total Ig. Serum samples reactive to either assay were considered positive.
ResultsWeighted overall seroprevalence was 6% (95%CI 3.9–8.3%). No association was observed between seropositivity and sex, age group or education level. Participants who reported black and brown skin color showed a 2.7 fold higher prevalence than people with white skin (p = 0.007). Among the 30 seropositive individuals, 14 (46.6%) reported no COVID-19 compatible symptoms in the past two weeks.
ConclusionThis study represents the first assessment of SARS-CoV-2 seroprevalence in the city of São Paulo and 6% is the baseline estimate of a series of population-based seroprevalence surveys. Serological screening using sound serological assays is the key tool to monitoring temporal and geographic changes in the spread of the virus through an important epicenter of the COVID-19 pandemic in Brazil. Ultimately, it may inform prevention and control efforts.
The spread of infection due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been ongoing worldwide since December 2019. In Brazil, the first laboratory-confirmed case of coronavirus disease 2019 (COVID-19) was reported on February 26, 2020. The patient was a 61-year-old male resident of São Paulo city, who had returned from a visit to Lombardy (Italy) a few days previously.1 On March 13, 2020, the Brazilian Ministry of Health declared that community transmission had been established as an upsurge of COVID-19 cases had been observed in multiple sites in the country.2 By May 23, 2020, approximately 350,000 laboratory-confirmed COVID-19 cases and 22,000 deaths had been reported in Brazil.3
The municipality of São Paulo, located in the Southeastern region of Brazil, is highly urbanized, with a population of approximately 11.6 million and a high level of socioeconomic inequality. The city has been an epicenter of the pandemic in the country and as of May 26, 2020, it had registered 425 cumulative cases per 100,000 inhabitants, compared to the national figure of 163 cases per 100,000.3,4
Knowledge about the magnitude of the SARS-CoV-2 epidemic is essential for future planning activities, such as formulation of public policies and control programs. It also underpins communication to the general population about the need for preventive measures.5 The number of laboratory-confirmed cases of COVID-19 and deaths reported to health authorities have been used as indicators of the extent of the epidemic. However, such data have not reflected the actual infection status in São Paulo, or Brazil, due to extremely low testing capacity and the high frequency of asymptomatic and oligosymptomatic SARS-CoV-2 infections which remain undetected. As seroprevalence surveys measure the proportion of the general population who have been infected and have produced anti-SARS-CoV-2 antibodies, they are useful for evaluating the dimension of the epidemic and to track its dynamic over time. Not only they do assess and monitor the spread of the virus regionally, but they are of paramount importance in informing vaccination plans.5,6
This study aimed to estimate the prevalence of anti-SARS-CoV-2 antibodies in a representative sample of the general adult population living in the six most affected districts in São Paulo city in the early epidemic phase. The potential associations between SARS-CoV-2 seropositivity and sociodemographic characteristics and self-reported COVID-19 compatible symptoms in the past two weeks were investigated.
Materials and methodsStudy design and sampling strategyThis cross-sectional population-based study was designed according to the World Health Organization (WHO) protocol for population-level COVID-19 antibody testing.7 The target population was individuals aged 18 years or older on the date of the study visit, residing in permanent private households, located within six administrative districts in the municipality of São Paulo. The districts were selected based on official data on the number of confirmed COVID-19 cases and suspected or confirmed COVID-19 deaths, released on April 17, 2020, by the Municipal Department of Health.8 The three districts with the highest number of confirmed cases per 100,000 inhabitants (Morumbi, Bela Vista, and Jardim Paulista) and the three districts with the highest number of suspected or confirmed deaths per 100,000 inhabitants (Pari, Belém, and Água Rasa) were included in the study.
A sample size of 500 individuals was planned to allow estimation of prevalence of seropositivity greater than 4% to be obtained under coefficients of variation of less than 30%, taking into account a design effect of 2 (deff = 2). Two-stage cluster sampling was used. In the first stage, 72 census tracts were selected using probability proportional to size, measured by the number of households. In the second stage, 16 households were randomly selected in each census tract. All residents aged 18 years or older who could understand the information provided by the research teams were eligible to participate. The sampling strategy did not allow to replace any household that could not be contacted or eligible adults who did not agree to participate in the study. A total of 1,152 households were selected and approached by the highly-trained survey team between May 4 and 12, 2020.
Data collectionThe fieldwork teams visited the selected households and invited the randomly selected residents to participate in the study. After signing an informed consent form, participants completed a structured questionnaire on sociodemographic characteristics and occurrence of COVID-19 compatible symptoms in the preceding two weeks. Questions included sex, age, skin color/race, and educational level measured by years of formal schooling. Self-reported skin color categories followed the Brazilian Institute of Geography and Statistics (IBGE) classification: white, brown, black, Asian, and indigenous.9,10 Shortly after the interview, a 5-ml blood sample was collected by venipuncture. . Other household members who were interested in participating in the study, followed the same procedures.
The study was approved by the Research Ethics Committee of Fleury Laboratory (CAAE 31032620.0.0000.5474) on April 27, 2020.
Laboratory phaseSamples were transported from the collection sites to the Serology Laboratory of Group Fleury where they were separated by centrifugation, aliquoted, and stored frozen until analysis. Within days of blood collection, all sera were tested for the presence of SARS-CoV-2 specific antibodies using the commercial kits MAGLUMI IgM 2019-nCoV and MAGLUMI IgG 2019-nCoV, chemiluminescence immunoassays (Snibe Diagnostics, Shenzhen, China). These assays were chosen because they were among the first available for use.
Chemiluminescence immunoassays for IgM and IgG detection were developed based on a double-antibody sandwich assay. The recombinant antigens contained the nucleoprotein and a peptide from the spike protein of SARS-CoV-2. The tests were conducted on an automated magnetic chemiluminescence analyzer (Axceed 260, by Bioscience Diagnostics, Tianjin, China) according to the manufacturer's instructions. The antibody titer was tested once per serum sample.
The performance of chemiluminescence immunoassays has been evaluated in the literature and sensitivity and specificity improve during the progression of infection.11–13 Tweenty days after symptoms onset, sensitivity for IgM is 100% and specificity 94.1% , and sensitivity for IgG is 100% and specificity 99.5% .11 To determine the reference values, samples from patients diagnosed with the COVID-19 RT-PCR test and samples from control groups such as blood donors, employees without flu symptoms vaccinated in 2020, patients that tested positive for other non-SARS-CoV-2 respiratory viruses, and carriers of other infectious diseases were used. After analyzing these samples, we used the following reference values: IgG: reagent >1.1 UA/mL, indeterminate 0.9–1.1 UA/mL, non-reagent <0.9 UA/mL; and IgM: reagent >1.0 UA/mL, indeterminate 0.7-1.0 UA/mL, non-reagent <0.7 UA/mL.
In September 2020, a sandwich electrochemiluminescence immunoassay (Elecsys® Anti-SARS-CoV-2 nucleocapsid total Ab ELISA, Roche Diagnostics, Rotkreuz, Switzerland)14,15 became available for serologic testing protocol. This test detects total antibodies against the SARS-CoV-2 nucleocapsid antigen with a sensitivity of >99% and specificity of 100%, according to the manufacturer. A reactive serum sample (cut-off ≥1.0) was considered a positive result.
A total of 517 individuals agreed to participate in the study (38.5% participation rate, considering non-contacts and refusals) and their serum samples were tested, using the Maglumi assay, shortly after data collection. In September 2020, stored sera of 463 participants were tested with the Roche assay, as 54 samples were not available due to laboratory problems. To evaluate whether this loss was biased in terms of seropositivity, we compared the estimate of seropositivity prevalence of the 463 retested samples measured by Maglumi assay only, with the estimate observed in the 517-sample analysis. Both results were the same, 4.7%. For the current analysis, our study population comprised 463 individuals whose serum samples had been measured using both tests.
Statistical analysisSeropositivity was the outcome of interest and was defined according to previously set laboratory parameters. Individuals whose sera were reactive to either Maglumi or Roche assays were considered seropositive. Indeterminate results were included as negative in the analyses.
Descriptive statistics consisted of the characterization of the study sample and anti-SARS-CoV-2 prevalence estimates with 95% confidence intervals (CI), by sociodemographic characteristics. Prevalence ratios were obtained for these characteristics using Poisson multivariate regression models. Frequency distributions of self-reported symptoms were compared between seropositive and seronegative individuals. All estimates were obtained considering the complex aspects of the design: sampling weights adjusted for response rates and post-stratification by age and sex, according to the district population structure in 202016 and sampling cluster.
Differences between percentages were assessed by survey-adjusted (Rao Scott correction) chi-square tests. A p-value <0.05 was defined as statistically significant. STATA version 14 (StataCorp, College Station, TX, USA) was used for all statistical analyses.
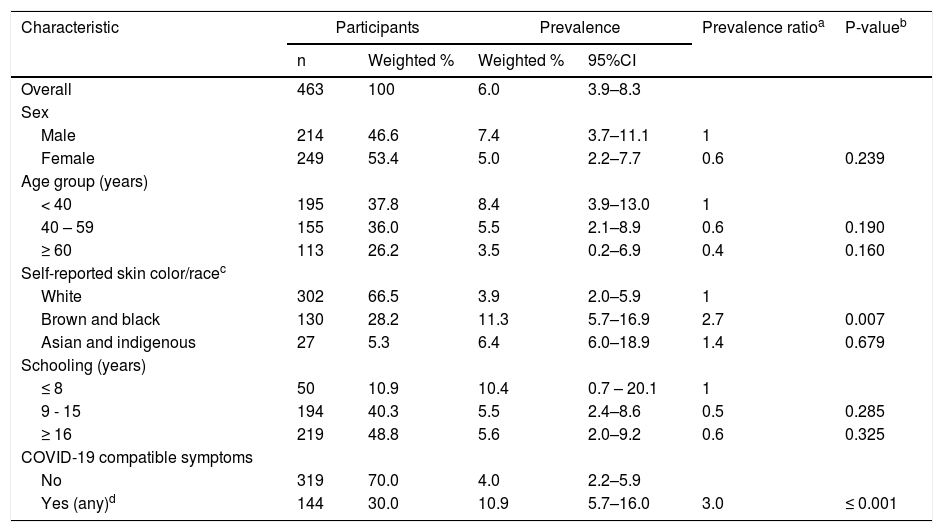
ResultsTable 1 shows weighted seroprevalence estimates and prevalence ratios by participant characteristics. The overall weighted seroprevalence for SARS-CoV- 2 was 6% (95%CI 3.9–8.3%), based on dual tests on the serum samples of 463 participants living in 299 households. Briefly, 46.6% were male, mean age 47.1 years (range 18-89), 66.5% self-reported as being white, and 48.8% had a university degree (16 years or more of schooling). In this sample, 46.6% of the 30 seropositive individuals did not report SARS-CoV-2 compatible symptoms in the preceding two weeks.
Prevalence and prevalence ratios of SARS-CoV-2 by sociodemographic characteristics and self-reported COVID-19 compatible symptoms, São Paulo City, Brazil, 2020.
| Characteristic | Participants | Prevalence | Prevalence ratioa | P-valueb | ||
|---|---|---|---|---|---|---|
| n | Weighted % | Weighted % | 95%CI | |||
| Overall | 463 | 100 | 6.0 | 3.9–8.3 | ||
| Sex | ||||||
| Male | 214 | 46.6 | 7.4 | 3.7–11.1 | 1 | |
| Female | 249 | 53.4 | 5.0 | 2.2–7.7 | 0.6 | 0.239 |
| Age group (years) | ||||||
| < 40 | 195 | 37.8 | 8.4 | 3.9–13.0 | 1 | |
| 40 – 59 | 155 | 36.0 | 5.5 | 2.1–8.9 | 0.6 | 0.190 |
| ≥ 60 | 113 | 26.2 | 3.5 | 0.2–6.9 | 0.4 | 0.160 |
| Self-reported skin color/racec | ||||||
| White | 302 | 66.5 | 3.9 | 2.0–5.9 | 1 | |
| Brown and black | 130 | 28.2 | 11.3 | 5.7–16.9 | 2.7 | 0.007 |
| Asian and indigenous | 27 | 5.3 | 6.4 | 6.0–18.9 | 1.4 | 0.679 |
| Schooling (years) | ||||||
| ≤ 8 | 50 | 10.9 | 10.4 | 0.7 – 20.1 | 1 | |
| 9 - 15 | 194 | 40.3 | 5.5 | 2.4–8.6 | 0.5 | 0.285 |
| ≥ 16 | 219 | 48.8 | 5.6 | 2.0–9.2 | 0.6 | 0.325 |
| COVID-19 compatible symptoms | ||||||
| No | 319 | 70.0 | 4.0 | 2.2–5.9 | ||
| Yes (any)d | 144 | 30.0 | 10.9 | 5.7–16.0 | 3.0 | ≤ 0.001 |
95%CI: 95% Confidence Interval.
SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; COVID-19, Coronavirus disease 2019.
No significant differences in seroprevalence were observed between sexes or age groups. People who reported brown and black skin color showed a 2.7-fold increase in the prevalence of SARS-CoV-2 antibodies, compared to those with white skin (p = 0.007). Although participants who reported eight or fewer years of schooling had higher seroprevalence than those who had 16 years or more of education (10.4% vs 5.6%), this difference was not statistically significant.
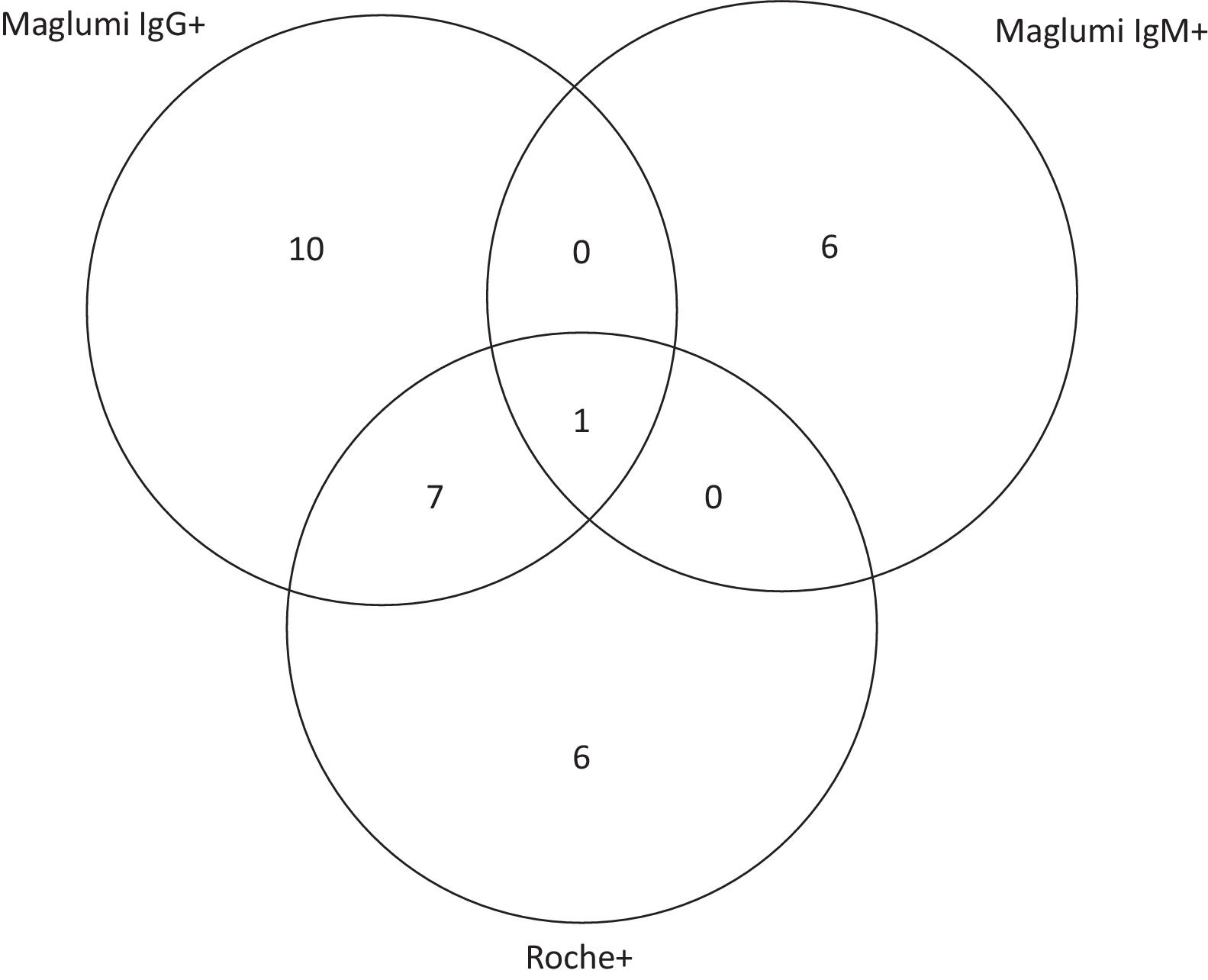
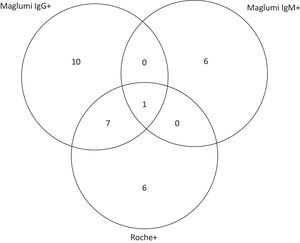
The distribution of positive laboratory results by Maglumi IgG, Maglumi IgM, or Roche tests is shown on the Venn diagram in Fig. 1. Among the 30 seropositive participants, eight were both Maglumi and Roche reactive; one of the seven IgM positives was also IgG reactive. The combination of the two antibody tests enhanced the detection rate by 28%, from 4.7% using Maglumi only, to 6.0%, using Maglumi and Roche.
Concordance (overlap area) of anti-SARS-CoV-2 reactivity between Maglumi IgG, Maglumi IgM and Roche in all seropositive participants (n = 30). Maglumi IgM, Maglumi SARS-CoV-2 chemiluminescence assay Immunoglobulin M (IgM); Maglumi IgG, Maglumi SARS-CoV-2 chemiluminescence assay Immunoglobulin G (IgG); Roche, Elecsys® Anti-SARS-CoV-2 electrochemiluminescence assay total immunoglobulin (Ig).
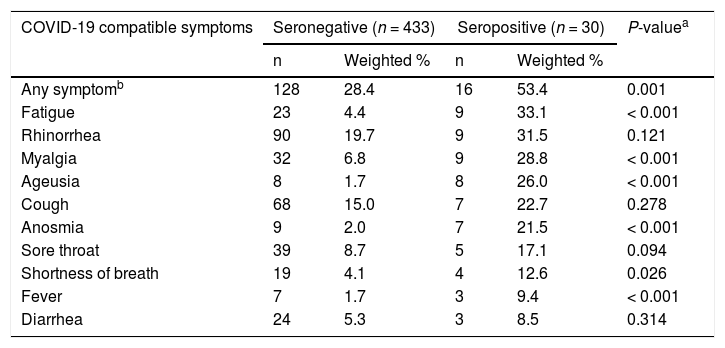
Frequencies of self-reported symptoms during the two-week period before the study visit stratified by SARS-CoV-2 seroreactivity are shown in Table 2. Seropositive individuals were significantly more likely to have experienced symptoms such as fever, shortness of breath, fatigue, myalgia, ageusia, and anosmia than seronegative participants. The most frequently reported symptoms among seropositive participants were fatigue (33.1%), rhinorrhea (31.5%), myalgia (28.8%), and ageusia (26%). None of the participants reported previous diagnoses of infection.
Self-reported COVID-19 compatible symptoms in the past 2 weeks by anti-SARS-CoV-2 seroreactivity, São Paulo City, Brazil, 2020.
| COVID-19 compatible symptoms | Seronegative (n = 433) | Seropositive (n = 30) | P-valuea | ||
|---|---|---|---|---|---|
| n | Weighted % | n | Weighted % | ||
| Any symptomb | 128 | 28.4 | 16 | 53.4 | 0.001 |
| Fatigue | 23 | 4.4 | 9 | 33.1 | < 0.001 |
| Rhinorrhea | 90 | 19.7 | 9 | 31.5 | 0.121 |
| Myalgia | 32 | 6.8 | 9 | 28.8 | < 0.001 |
| Ageusia | 8 | 1.7 | 8 | 26.0 | < 0.001 |
| Cough | 68 | 15.0 | 7 | 22.7 | 0.278 |
| Anosmia | 9 | 2.0 | 7 | 21.5 | < 0.001 |
| Sore throat | 39 | 8.7 | 5 | 17.1 | 0.094 |
| Shortness of breath | 19 | 4.1 | 4 | 12.6 | 0.026 |
| Fever | 7 | 1.7 | 3 | 9.4 | < 0.001 |
| Diarrhea | 24 | 5.3 | 3 | 8.5 | 0.314 |
COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
This study estimated an overall weighted prevalence of antibodies against SARS- CoV-2 of 6% in a representative sample of residents of the six most affected districts in São Paulo city, in the initial phase of the pandemic, i.e., 10 weeks after the first case of COVID-19 was confirmed. Combining two tests, Maglumi and Roche, resulted in a 28% improvement in sensitivity, thus a more accurate seroprevalence estimate. Differences in the isotypes of antibodies, in the composition of viral antigens and in the technology may partially explain this finding.17,18
At the time of the current study, two other seroprevalence studies were performed in the city of São Paulo.19,20 and although there were differences in the methodolog, their estimates are similar to ours. One, based on an 826-convenience sample of blood donors and using the Abbott SARS-CoV-2 IgG chemiluminescence assay (Abbott, Chicago, USA) reported a weighted prevalence of 5.1% (95%CI 3.4–7.2%).19 The other was part of a nationwide population-based serosurvey and, in São Paulo, it was based on 212 participants. The authors found a seroprevalence of 3.3% (95%CI 1.3–6.7%) using a rapid capillary blood test (Wondfo Biotech One step COVID-19, Guangzhou, China).20 Their study probably underestimated the true SARS-CoV-2 seroprevalence due to use of lateral flow immunoassays that have lower sensitivity than fully automated assays such as those used in both our study and the blood donors’ study.17,21,22 Our estimate might reflect the upper limit of the SARS-CoV-2 seroprevalence in the city considering that we studied the districts with the highest number of cases and deaths at the time the pandemics started in São Paulo.
Few other studies in Brazil estimated the seroprevalence of SARS-CoV-2 in the general population based on household surveys or blood donors around the period of our data collection. In Espirito Santo, in the Southeast region, the estimate was 2.1% (95%CI 1.7–2.5%),23 in Rio Grande do Sul, in the South, 0.2% (95%CI 0.4–2.0),24 and in Manaus, in the North, 37.4% (95%CI 33–42%).19 These prevalence rates, as well as the results of our study, may not be easily compared because they represent distinct moments of the pandemic in specific locations. Moreover, the use of different laboratory tests, different characteristics of the populations, and different study designs might be responsible for the observed prevalence heterogeneity.
Also, in April and May 2020, seroprevalence population-based surveys were performed in several other countries. The seropositivity rates were 10.8% in Geneva (Switzerland),25 4.6% in Spain,26 14% in New York State (USA),27 and 0.9% in Iceland.28 A modeling study estimated that 4.4% of the French population had been infected as of May 2020.29 As discussed before, the heterogeneity in prevalence estimates may be explained by the substantial differences between studies in terms of laboratory testing methods and sampling strategies, making comparisons difficult. Additionally, countries and regions faced different stages of the epidemic at different times and seroprevalence estimates not only change over time, but also vary according to adherence to a reduction of social proximity, home quarantine, and to the overall behavior of the population.30,31
Besides assessing the magnitude of cumulative infections in the chosen areas of São Paulo city, we looked at possible associations between sociodemographic factors and SARS-CoV-2 seroreactivity. No difference in seroprevalence was found between male and female participants; likewise, several reports indicated that seroprevalence is not significantly associated with sex.30,31 Prevalence rates were similar amongst age groups, in line with findings reported in a meta-analysis study.31 The infection rate was lower among participants with white skin than those with black and brown skin color, as also found in the Brazilian nationwide survey20 and in other international studies.31 SARS-CoV-2 seroprevalence depends on isolation adherence, which in turn, varies according to multiple socioeconomic aspects such as employment, transportation, income, housing, population density, health, and education. Inequality and exposure to poverty are historically related to race and black and brown skin color in Brazil10 and the concept of poverty-related diseases might apply to COVID-19.32
Previous reports have linked SARS-CoV-2 infections with no or minor symptoms.33-35 In our study, 46.6% of seropositive individuals reported no symptoms during the two weeks before the study visit. In line with findings from other studies,20,26 several SARS-CoV-2 compatible symptoms such as anosmia, ageusia, fatigue, myalgia, shortness of breath, and fever were significantly associated with seropositivity. It is noteworthy that SARS-CoV-2 compatible clinical symptoms and signs of mild infections remain poorly documented in Brazil.
An accurate estimate of the number of infected people requires an accurate measure of both documented and undocumented infections. The strengths of the current research are that it is the first household population-based serosurvey in Brazil to assess the prevalence of anti-SARS-CoV-2 immunoglobulins based on two validated, fully automated sensitive tests, the Maglumi and Roche immunoassays. These tests, together with the probabilistic sampling design, provided a clear picture of the extent of human exposure to SARS-CoV-2 in the studied regions. Also, although we have only surveyed six districts in the city, the sample is truly representative of the adult general population and reflects the heterogeneity in susceptibility or exposure to infection across population groups.
Our study has some limitations. First, due to its cross-sectional design, causal associations cannot be established. Second, refusal bias may have been introduced as a result of non-response which occurred mainly due to non-cooperation by the managers of some residential blocks and residents’ unwillingness to donate blood. Third, we have not included children or young people under 18 years due to logistic restrictions. Fourth, the statistical power may have been insufficient to detect differences between subgroups, such as education level. Last, the generalizability of the results of this study is somewhat limited as we only included six districts in São Paulo city at the beginning of the pandemic in the region. However, we believe that these issues have not affected our findings significantly, considering that they may have led to underestimation and overestimation, counterbalancing their effects on the prevalence estimates.
In conclusion, this study represents the first assessment of SARS-CoV-2 seroprevalence in the city of São Paulo, providing a baseline estimate. The low prevalence of 6% indicated that the COVID-19 pandemic was in its early phase, meaning that most of the population was susceptible to infection. This is the initial study of a series of consecutive household population-based seroprevalence surveys of adults aged 18 years or older in the municipality of São Paulo. The ongoing surveys use a similar methodology and testing strategy, based on random selection of households each time. Monitoring the progress of the pandemic throughout the entire city is an essential strategy to inform transmission control decisions and to appraise and update the vaccination plan for São Paulo city.