Aptamers are short single-stranded RNA or DNA oligonucleotides that are capable of binding various biological targets with high affinity and specificity. Their identification initially relies on a molecular process named SELEX (Systematic Evolution of Ligands by EXponential enrichment) that has been later modified in order to improve aptamer sensitivity, minimize duration and cost of the assay, as well as increase target types. Several biochemical modifications can help to enhance aptamer stability without affecting significantly target interaction. As a result, aptamers have generated a large interest as promising tools to compete with monoclonal antibodies for detection and inhibition of specific markers of human diseases. One aptamer-based drug is currently authorized and several others are being clinically evaluated. Despite advances in the knowledge of parasite biology and host–parasite interactions from “omics” data, protozoan parasites still affect millions of people around the world and there is an urgent need for drug target discovery and novel therapeutic concepts. In this context, aptamers represent promising tools for pathogen identification and control. Recent studies have reported the identification of “aptasensors” for parasite diagnosis, and “intramers” targeting intracellular proteins. Here we discuss various strategies that have been employed for intracellular expression of aptamers and expansion of their possible application, and propose that they may be suitable for the clinical use of aptamers in parasitic infections.
Aptamers are DNA or RNA oligonucleotides with a unique tridimensional structure that allows them for interacting with a specific target with high affinity and specificity. The term “aptamer” is derived from the Latin word “aptus” meaning “to fit” and a Greek word “mers” meaning “particle”.1 Although aptamers were first described as artificial molecules, they were later found as natural components of riboswitches that affect transcription or translation.2,3 The term aptamer can also design a peptide with protein-binding properties; however, here we will only focus on oligonucleotide molecules.
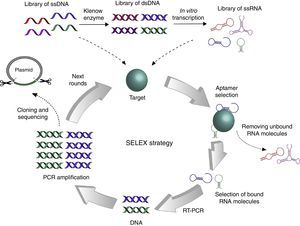
The strategy to obtain specific aptamers is called SELEX for its acronym in English Systematic Evolution of Ligands by EXponential enrichment. Two independent groups in the United States simultaneously published this method, one at the University of Colorado, and one at Harvard University (Fig. 1).1,4 Briefly, the SELEX strategy starts with a pool of RNA or DNA of 1014 to 1015 random sequences that interact with a target protein. The oligonucleotides that bind the target are retrieved, while the others are discarded. The selected oligonucleotides are PCR-amplified (or RT-PCR amplified in the case of RNA aptamers) to perform additional rounds of selection until more than 90% of aptamers interact with the target. After that, cloning and sequencing of aptamers is performed to identify a consensus motif between selected aptamers. This universal process must be standardized for the selection of each target-specific aptamer by varying the target concentration, the number of rounds, and the stringency of the binding conditions, among others.
Graphical representation of the SELEX strategy. The initial library of ssDNA may be used immediately for interaction with the target, while the generation of ssRNA library requires additional steps for synthesis of complementary strand by Klenow enzyme and in vitro transcription. Different strategies can be used to evaluate the interaction between oligonucleotides and the target, but the objective always remains to select bound molecules and discard unbound molecules. Then, selected aptamers are PCR amplified to start a new round of SELEX. When more than 90% aptamers recognize the target, DNA fragments are cloned into a plasmid to be sequenced.
The original SELEX strategy has evolved significantly and several modified techniques have been developed. For example, it is possible to use whole living cells as targets to isolate aptamers against unspecified determinants on cell surface.5 This cell-SELEX strategy that targets proteins in their native conformation has been proved to be worthwhile to identify relevant markers for cancer and other diseases; importantly, it is also useful in the case of human pathogens, such as bacteria, viruses, and protozoan parasites.6,7 The blended-SELEX consists in incorporating a no-nucleic acid component into the nucleic acid library to lead the aptamers towards a specific region of the target and increase its functionality.8 The covalent SELEX or cross-linking SELEX or Photo-SELEX uses a RNA library with uracil analogs (5-iodouracil (5-IU) or 5-bromouracil (5-BrU)) to select aptamers with high affinity and specificity that can be UV-cross-linked to the target protein for biochemical and structural studies of nucleic acid–protein interactions and therapeutic applications.9,10 The mirror-image SELEX or Spiegelmer technology generates DNA or RNA aptamers that contain L-2′-deoxyribose or L-ribose units, and are therefore resistant to nuclease degradation, which improves the in vivo stability.11 In the tailored-SELEX method, the fixed primer regions flanking the 20–40nt long recognition region, are shortened from 15–25nt to 4–6nt after the amplification of the library.12 When species cross-reactivity is desired, the use of homologous targets from two distinct species in the toggle-SELEX allows the selection of aptamers that have the same efficacy in animal models and clinical trials.13 In the capillary electrophoresis SELEX or CE-SELEX protocol, aptamers interacting with the target molecule are isolated based on their differential rate of migration, which significantly minimizes the number of selection rounds and therefore the duration and cost of the process.14–16 The use of magnetic beads-conjugated targets together with microfluidics technology represents the principle of the chip-based micromagnetic SELEX or M-SELEX for the rapid generation of aptamers with high affinity and specificity.17,18 Other methods combine the SELEX strategy to atomic force microscopy (AFM-SELEX) or surface plasmon resonance (SPR-SELEX) to select aptamers with higher target affinity.19,20 The development of microarrays containing tens of thousands of probes has the potential to speed up the identification of highly specific aptamers.21 These and other variants of SELEX greatly contributed to the promising use of aptamers in human health.
Aptamers for diagnostics, therapeutics, and drug developmentSince the publication of the SELEX protocol in 1990, many efficient aptamers have been described and a large number of studies have revealed their superiority for diagnostics, therapeutics, and drug development, in comparison with other tools, essentially monoclonal antibodies that are widely used for detection and inhibition of target molecules in the biomedical field (Table 1). One of the main advantages of aptamers is that they can be selected and functionally characterized in less than 2 years, while it requires much more time to develop antibodies. Aptamers are chemically synthesized, which facilitates their commercialization as therapeutic tools. Moreover, their nucleic acid composition makes them thermostable molecules and allows their production and conservation at room temperature. All these characteristics largely contribute to the low-cost benefit of aptamers production, which represents a significant criterion for pharmaceutical and biotechnological industries. Antibodies must be targeted against immunogenic proteins, while aptamer can be designed against almost any kind of molecules, including proteins, ions, or whole cells, without any risk of evoking a negative immune response. Moreover, a number of studies showed that dissociation equilibrium constants (Kd) of aptamers are in the range of nM to pM, indicating that aptamers have a similar or a greater affinity for their target than antibodies. In addition, aptamers can be chemically modified to improve their efficiency. The main disadvantage of aptamers is that they can be attacked by cellular nucleases. However, several changes, either in the structure of the sugar component or the organic base, can make them resistant to nuclease degradation.22
Similarly to antibodies, aptamers can be used to detect specific molecules in diagnostic applications, as well as to act as targeting and therapeutic agents for disease treatment. They can also be used to purify native or recombinant proteins for further characterization. As a consequence, a number of pharmaceutical companies have promoted the development of aptamers and clinical studies have rapidly been initiated. In 2004, the US FDA (United States Food and Drug Administration) approved for the first time the use of an aptamer as a therapeutic agent for age-related macular degeneration (AMD) in human. Pegaptanib sodium/Macugen (Eyetech Pharmaceuticals/Pfizer) is an RNA aptamer directed against vascular endothelial growth factor (VEGF)-165, which is primarily responsible for pathological ocular neovascularization and vascular permeability. VEGF is a major angiogenesis inducer through binding to its receptors (VEGFR1 and VEGFR2) and activation of downstream signaling pathways.23,24 Using the SELEX strategy, Jellinek et al. isolated six related families of aptamers that target the heparin-binding site on VEGF and inhibit the binding to its cellular receptors in vitro.25 Then, incorporation of 2′-NH2-pyrimidines into aptamers, substitution of 2′-OMe for 2′-hydroxyl of purines, and incorporation of phosphorothioate-linked polydeoxythymidine caps at both ends resulted in an anti-VEGF aptamer with an enhanced endonuclease resistance and binding affinity (Kd=0.14nM).26 Finally, integration of 2′-F-substituted pyrimidines and addition of a 5′-linked 40-kDa polyethylene glycol moiety led to the development of a high-affinity (Kd=200pM) and high stability anti-VEGF aptamer that was designated as Pegaptanib.27 After satisfactorily completing the toxicological and pharmacological pertinent assays, Pegaptanib was tested in patients with AMD and diabetic macular edema, barely a decade after the first published report.28 Ten years later, different randomized controlled trials confirmed that Pegaptanib is safe and effective for the treatment for ocular vascular diseases.29
Currently, 10 aptamers are in distinct phases of undergoing clinical trials in patients with neovascular AMD (E10030 and ARC1905, Ophthotech), coronary artery disease (RB006, Regado Bioscience; NU172, ARCA Biopharma), von Willebrand's disease (ARC1779, Archemix Corp), hemophilia (ARC 19499; Archemix), type II diabetes mellitus/renal impairment/nephropathy/lupus nephritis (NOX-E36, Noxxon Pharma), and cancer (AS1411, Antisoma; NOX-A12, Noxxon Pharma).30
Tools for protection and targeted delivery of aptamers into cellsAs described above, aptamers targeting cell surface markers or secreted proteins are particularly interesting for diagnosis and treatment of human diseases. Aptamers can also be designed against proteins that are located either in the cytoplasm or the nucleus of cells. Because of their intracellular mode of action, these aptamers have been designed as “intramers”.31 Notably, incorporation of artificial aptamers in natural systems can be used to alter the different steps of gene expression. Of particular interest for this review, the development of aptamers that modify functionality of proteins involved in key cellular and molecular processes, such as gene expression regulation, genome stability, as well as energy metabolism and cytoskeleton, represents a novel line of investigation for the control of human parasites.
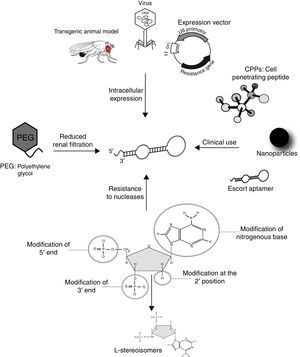
Generally, aptamers are selected based on their high specificity and affinity for a selected target in in vitro experiments. To efficiently bind intracellular proteins in vivo, DNA and RNA aptamers have to face two main problems: (i) they have to “survive” inside the cell, organ or organism; (ii) they have to reach their target inside the cells. Several strategies have been developed for the therapeutic use of oligonucleotides (Fig. 2). As low-molecular weight molecules, wild-type aptamers are eliminated from the bloodstream in several minutes or tens of minutes, as a result of exo- and/or endo-nuclease degradation, as well as kidneys clearance. Aptamer modification by addition of specific chemical groups, such as polyethylene glycol at the 5′-end, enhances half-life in vivo, although it may affect binding affinity or specificity toward the target. Substitution with nucleotides modified at 2′ sugar position, such as 2′-amino pyrimidines, 2′-fluoropyrimides, 2′-O-methyl purines and pyrimidines, during the SELEX protocol, also confers greater stability and pharmacokinetic lifetimes to aptamers without affecting target affinity. Another approach consists in using L-enantiomer of deoxyribose or ribose to synthesize the so-called Spiegelmers that are resistant to nuclease digestion.32,33
Modifications of aptamers for their use as diagnosis and therapeutics tools. The half-life of aptamers in serum can be increased by conjugation to PEG to prevent renal elimination; in addition, chemical modifications at 5′ and 3′ ends, and 2′ position of the sugar component, as well as the use of L-steroisomers and modified nitrogenous base, make them resistant to nuclease degradation. Virus, transgenic animal models and expression vectors have been shown to be effective for intracellular disposition of aptamers. The conjugation to a cell penetrating peptide, a nanoparticle, or an escort aptamer, may represent suitable strategies for the clinical use of intramers in parasite infections.
To overcome the problem of crossing cellular and nuclear membranes, it is possible to use specific vectors to express aptamers inside the cell. For example, Good et al. used an RNA polymerase III-dependent expression vector to produce an intramer that blocks the nuclear Rev protein involved in exportation of viral RNA to cytoplasm, which inhibited HIV-1 production in cell culture models.34 Using the same vector, Thomas et al. targeted the yeast RNA polymerase II in nucleus, affecting cell proliferation.35 Alternatively, Mayer et al. used an intramer expression system based on the infection of T-cells with recombinant vaccinia viruses, whose complete life cycle occurs in the cytoplasm of the host cells. The T7 RNA polymerase-controlled expression of M69 aptamer that targets the Sec7 domain of protein cytohesin-1 (cyh1), a protein involved in the control of membrane-associated events and the remodeling of the cytoskeleton, resulted in a cell-spreading deficiency and a dramatic reorganization of F-actin distribution in leukocytes.36 Another strategy is based on the development of transgenic animal models. Shi et al. created a transgenic Drosophila melanogaster that expresses an intramer against the B52 splicing protein, which produces a 50% reduction in the development of adult transgenic flies.37 On the other hand, the three hybrid assay has been applied to evaluate the effect of a RNA aptamer that targets the nuclear transcription factor NF-κB.38
Development of intramers as anti-parasite moleculesAs mentioned above, aptamers represent a promising approach for the control of parasitic diseases that largely compromise human health worldwide. At the beginning of the twenty-first century, hundreds of million people in underdeveloped countries are affected by Trypanosomatida parasites, such as Trypanosoma brucei and Trypanosoma cruzi that cause the African trypanosomiasis (sleeping sickness) and American trypanosomiasis (Chagas disease), respectively, as well as various species of Leishmania that produce cutaneous, mucocutaneous, and visceral leishmaniasis.39 Malaria caused by several Plasmodium spp., mainly Plasmodium falciparum and Plasmodium vivax, is another important parasitic disease with an estimated 600,000 deaths and 200 million cases annually.40 In the last decade, advances in the knowledge of parasite biology and host–parasite interactions have been obtained by the use of new technologies, such as “omics” strategies, promoting the development of better diagnostic and therapeutic methods. However, these parasites still threaten human life worldwide and there is an urgent need for the development of new strategies for drug target discovery and novel therapeutic concepts.
As described above, aptamers can recognize a specific target with high affinity, which is of particular interest for the development of new methods for the detection of human parasites. Thus, SELEX strategy allowed the discovery of aptamers that can be used as pathogen “aptasensors” to specifically identify T. cruzi, and Plasmodium in blood,41–44Plasmodium-infected red blood cells,45 as well as Leishmania in sandflies.33 Some works also showed that aptamer-based detection of disease biomarkers can be applied to assess efficacy of new drugs against Chagas disease.46
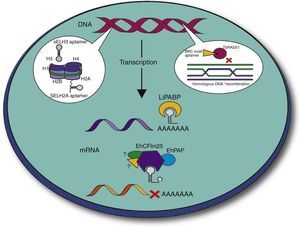
Aptamers can also inhibit protein functions in vitro making them relevant tools for the treatment of parasitic infections. In this context, several groups have been interested in the search for aptamers against these protozoan parasites, since they represent stable, efficient and economic tools. Several strategies have been addressed to block the interaction between the parasite and the host. For example, Barfod et al. reported RNA aptamers targeting the P. falciparum erythrocyte membrane protein 1 that enables parasite adherence to endothelial vessels and non-infected erythrocytes.47 Notably, other groups used the SELEX strategy to isolate intramers targeting cytoplasmic and nuclear parasite proteins in order to attack the parasite intracellularly (Fig. 3). During the last years, the group of Prof. Gonzalez has used SELEX to select and characterize aptamers that recognize specific L. infantum nuclear proteins, namely histones. In kinetoplastid parasites, amino acid sequence of histones is highly divergent at the N- and C-terminal regions, which make them attractive targets.48 From three rounds of selection, Ramos et al. isolated two pools of DNA sequences named SELH2A and SELH3, which target recombinant LiH2A and LiH3, respectively, with high affinity (Kd=0.94±0.19nM and Kd=2.0657±0.652nM, respectively). These ssDNA sequences were highly specific since they did not recognize other Leishmania proteins neither other histones, according to Enzyme-Linked OligoNucleotide Assay (ELONA), slot blot and Western blot analyses. Notably, two sequences identified as AptLiH2A#1 and AptLiH2A#2 showed the highest affinity for LiH2A, probably due to the presence of a G-quadruplex structure and complex secondary structures (protruding loops and stems), respectively. Both aptamers were able to detect recombinant LiH2A in a concentration dependent manner with Kd=0.96±0.17nM and 1.16±0.28nM, respectively, which corresponds to the best range of most of the aptamers previously described. Mapping of H2A–aptamer interactions suggested that these ssDNA aptamers recognize amino acid residues that constitute a pocket region that is accessible when H2A is forming part of the nucleosome. Interestingly, both aptamers were able to identify recombinant and endogenous H2A and H3 proteins from parasite lysates, demonstrating their potential use as purification and diagnostic tool.49–51
Development of intramers against parasite proteins. Several groups of investigation have reported intramers that recognize key proteins in molecular processes that are essential for parasite survival. Selected targets included histones H3 and H2A that contribute to DNA packaging49–51 and the poly(A) binding protein (PABP) involved in mRNA 3′ end formation, stability and translation53 in Leishmania, as well as the RAD51 protein involved in homologous recombination in Trypanosoma.56 In our group of investigation, we are currently developing intramers that target the CFIm25 protein that plays an important role in the cleavage and polyadenylation of mRNA 3′ end in E. histolytica (Ospina-Villa et al., unpublished data).
Another feature of trypanosomatids parasites is that the expression of stage-specific genes to survive within the insect vector and the vertebrate host is highly regulated through posttranscriptional maturation events.52 Considering the relevance of the poly(A)-binding protein (PABP) in translational initiation and termination, and in mRNA turnover in eukaryotic cells, the same group of investigators focused on the identification of aptamers targeting the LiPABP protein. From four rounds of SELEX, they selected the SELLiPABP population that recognizes the recombinant LiPABP with high affinity (Kd=3.87±0.67nM) and sensitivity according to ELONA and slot assays. The three aptamers (ApPABP#3, ApPABP#7 and ApPABP#11) with the highest affinity for rLiPABP (Kd=5.4±1.1nM, Kd=6.0±2.6nM and Kd=10.8±2.7nM, respectively) were able to detect LiPABP from only 2500 parasites, which is a promising result for the development of a sensitive method that would detect LiPABP in both promastigote and amastigote forms of Leishmania. Interestingly, these aptamers have different nucleotide sequences and predicted secondary structures, indicating that each of them could recognize different “aptatopes” on LiPABP surface. Results from poly(A)-sepharose binding assays showed that ApPABP#11 is the only molecule that is able to reduce the poly(A) binding activity of recombinant LiPABP (30%) and ectopically overexpressed myc-LiPABP (80%). This suggested that ApPABP#11 may target the poly(A) binding site, probably affecting protein translation in Leishmania, making it a promising therapeutic tool. On the other hand, ApPABP#3 and ApPABP#7 were more efficient ligands to purify recombinant LiPABP from bacterial extract.53
Trypanosma sp. parasites use homologous recombination (HR) of DNA to perform antigenic variation of Variant Surface Glycoprotein (VSG) and escape the host immunity system, and control DNA damage resulting from stress during transmission stages.54,55 One of the factors involved in HR is the multifunctional scaffolding protein BRCA2 that is considered as a potential pharmacological target due to its structural differences with the human homologue. Notably, TbBRC2 interacts with the main recombinase TbRAD51 through BRC repeats. By using a doxycycline-inducible system in T. brucei cells, the authors demonstrated that the in vivo production of an aptamer containing a single BRC motif leads to an increase in DNA damage sensitivity and inhibition of parasite proliferation. These interesting results revealed that it is possible to develop strategies to deliver intramers, in this case a peptide aptamer, into the nucleus to effectively sequester its target and affect parasite survival.56
In our group of investigation, we are interested in the study of pre-mRNA 3′ end processing in Entamoeba histolytica, the protozoan parasite responsible for human amebiasis, which causes intestinal dysentery and hepatic abscesses worldwide, resulting in 70,000 to 100,000 deaths per year.57 We previously identified the putative polyadenylation machinery in E. histolytica and showed that the 25-kDa subunit of the Cleavage Factor Im (EhCFIm25) is an RNA binding protein that interacts with the poly(A) polymerase EhPAP.58,59 We also demonstrated that amino acid residues Leu135 and Tyr236 are essential for RNA interaction.60 In human, CFIm25 is a key factor for mRNA 3′ end formation. CFIm25 down-regulation produced alterations in the selection of poly(A) sites, which affected the size of mRNA 3′ ends in HeLa cells.61 Moreover, blocking of CFIm25 by a specific RNA aptamer produced alteration in the recruitment, cleavage and polyadenylation reactions.62 These data led us to evaluate the relevance of EhCFIm25 for E. histolytica gene expression and survival. Our results revealed that EhCFIm25 inhibition by bacterially expressed dsRNA and soaking experiments, affects growth and polyadenylation reaction in vitro, confirming the relevance of this protein for parasite survival. Therefore, we used the SELEX protocol to identify aptamers that selectively recognize EhCFIm25 but not the human homologue (Ospina-villa et al., unpublished data). Taking advantage of the high phagocytosis capacity of E. histolytica cells, we are currently developing a strategy to introduce these aptamers within trophozoites and block endogenous EhCFIm25. In such a configuration, our intramers would serve as universal regulators affecting mRNA polyadenylation, stability and translation, as well as other mRNA-related processes. We hypothesize that alterations in protein synthesis would have a general impact on trophozoites affecting their proliferation and survival, and therefore their virulence properties.
Strategies for the clinical use of intramers in parasite infectionsOnce intramer has been proved to efficiently block its target and affect parasite survival in cell cultures, another difficulty relies on its clinical application as an anti-parasite drug in an animal model and human. Importantly, several questions have emerged: will aptamers selected from their affinity for recombinant proteins efficiently inhibit their target in a whole organ or tissue? What is the best route of administration for aptamers reach their target within the parasite? Would aptamers also affect host proteins? How is the biodistribution of aptamers within the organism? How to make sure that aptamers do not degrade within the organism? Although some authors claim that aptamers targeting intracellular proteins are not good therapeutic candidates for parasite control, some data emerging from the use of functionalized aptamers in animal models and other contexts suggest the feasibility of the clinical use of intramers in parasitic infections (Fig. 3).
Several groups have used an in vivo SELEX protocol to isolate aptamers that localized into a specific organ or tissue, for example liver and brain, after injection of the library of nucleic acids into the peripheral vasculature of mice.63,64 This indicated that aptamers can migrate, probably through blood, to reach certain parts of the body. We propose that a similar method may be used to identify those aptamers that have an enhanced penetration into target cells, i.e. the parasite itself or host cells that harbor the parasite of interest.
On the other hand, some groups have developed aptamer-functionalized targeted drug delivery systems based on the conjugation with small molecules or a nanomaterial (Fig. 3). For example, Huang et al. conjugated the drug doxorubicin (DOX) to the Sgc8 DNA aptamer that specifically binds to protein tyrosine kinase 7 (PTK7) to efficiently inhibit the proliferation of CCRF-CEM (T-cell acute lymphoblastic leukemia, T-cell ALL) cells.65 In another work, they used gold nanoparticles (AuNPs) to improve the antitumor efficacy of sgc8c-DOX conjugate against targeted cancer cells.66 The sgc8 aptamer was also coupled to the surface of DOX containing liposomes to facilitate target cell binding and the consequent cell proliferation inhibition by DOX.67 Many examples of efficient so called “escort” aptamers using other inorganic (nano-scale iron oxides, carbon nanomaterials, mesoporous silica nanoparticles, quantum dots) and organic (poly(lactide-co-glycolic acid) nanoparticles, polymeric micelles, dendrimers, serum albumin nanoparticles, DNA) nanomaterials have been described for targeted cell delivery and specific cell detection.68 All these studies indicated that combining the advantages of nanomaterials with the high affinity and specificity of aptamers, greatly improved drug delivery. Therefore, conjugation of aptamers with a nanoparticle may also represent a suitable strategy to guarantee that aptamers efficiently reach their parasite target within the host.
Escort aptamers with high affinity to certain cell-surface proteins, have also been used as vehicles for targeted delivery of nucleic acids into cells of a definite type. For example, escort aptamers raised against the prostate-specific membrane antigen (PSMA) have been successfully designed to deliver small interfering RNAs (siRNAs) into tumor cells expressing this antigen on their surface.69 One can imagine that a chimeric RNA construct built from an aptamer raised against a specific membrane determinant and an aptamer raised against an intracellular target would permit the effective and specific delivery of intramer into parasites or infected cells through receptor-dependent endocytosis.
Cell penetrating peptides (CPA) are short amphipathic and cationic peptides that have the ability to cross cell membranes. They represent powerful transport vector tools for the intracellular delivery of a large variety of cargoes, such as plasmid DNA, oligonucleotides, siRNAs, proteins and peptides, contrast agents, drugs, as well as various nanoparticles, both in vitro and in vivo. Notably, several works have shown that CPA have the potential to facilitate aptamer entry into target cells.70–72 Therefore, it is tempting to propose the design of an aptamer-CPA conjugate that would allow the translocation of the aptamer into a relevant form of the parasite in the host or into parasite infected cells.
ConclusionAll the studies published so far indicate that RNA and DNA aptamers have a huge potential of applications in the biomedical field. The numerous advantages of aptamers in terms of production, stability, lack of immunogenicity, target selection and chemical modification make them very attractive molecules for detection, isolation and inhibition of markers of human diseases. Moreover, the rapid advances in the development of new SELEX-based strategies have allowed the selection of aptamers with higher affinity and specificity for their target. Over two decades, the development of a large number of SELEX variants and aptamers modifications has contributed to the improvement of libraries for the selection of highly specific aptamers. They allowed aptamers enter the cell environment, regulating their production and detection. They also took advantages of modern technologies such as wide analyses and bioinformatics. As a result, aptamers have become a promising alternative as therapeutic agents in modern molecular medicine. Today, many pharmaceutical and biotechnological industries bet on aptamers to replace antibodies or improve antibodies properties in the near future. In the field of parasitology, recent works have demonstrated the interest of aptamers for pathogen diagnosis and control, as well as the evaluation of drug efficacy. Notably, some interesting emerging directions suggest the feasibility of the clinical use of intramers in parasitic infections. In our opinion, a tempting strategy is to conjugate intramers to a nanoparticle, an escort aptamer, or a cell penetrating peptide, which may be used for intracellular targeting to bring about some therapeutic effect. Further works on functionalized intramers are required to expand the possibilities of inhibiting cytoplasmic and nuclear parasite proteins to alter pathogen survival and virulence, which would benefit the clinical use of aptamers for the control of parasitic diseases.
FundingThis work was supported by Mexican grants from CONACyT (178550) and SIP-IPN (20160801). ERM, AZC and LAM are supported by COFAA-IPN and EDI-IPN. JDOV is a scholarship recipient from Mexican BEIFI-IPN and CONACyT programs.
Conflicts of interestThe authors declare no conflicts of interest.