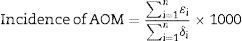To estimate acute otitis media incidence among young children and impact on quality of life of parents/caregivers in a southern Brazilian city.
MethodsProspective cohort study including children 0–5 years of age registered at a private pediatric practice. Acute otitis media episodes diagnosed by a pediatrician and impact on quality of life of parents/caregivers were assessed during a 12-month follow-up.
ResultsDuring September 2008–March 2010, of 1,136 children enrolled in the study, 1074 (95%) were followed: 55.0% were ≤2 years of age, 52.3% males, 94.7% white, and 69.2% had previously received pneumococcal vaccine in private clinics. Acute otitis media incidence per 1000 person-years was 95.7 (95% confidence interval: 77.2–117.4) overall, 105.5 (95% confidence interval: 78.3–139.0) in children ≤2 years of age and 63.6 (95% confidence interval: 43.2–90.3) in children 3–5 years of age. Acute otitis media incidence per 1000 person-years was 86.3 (95% confidence interval: 65.5–111.5) and 117.1 (95% confidence interval: 80.1–165.3) among vaccinated and unvaccinated children, respectively. Nearly 68.9% of parents reported worsening of their overall quality of life.
ConclusionAcute otitis media incidence among unvaccinated children in our study may be useful as baseline data to assess impact of pneumococcal vaccine introduction in the Brazilian National Immunization Program in April 2010.
Acute otitis media (AOM) is a common bacterial infection affecting children at least once before their second birthday, and is a leading cause of health care visits and antibiotic prescription worldwide.1,2 Globally, 709 million AOM cases are estimated to occur every year, of which 51% are seen in children ≤5 years of age.3 In Latin America and the Caribbean, 11.7–360 AOM episodes per 1000 children <5 years of age are estimated to occur annually.4 We report the incidence of AOM among children ≤5 years of age and impact on quality of life (QoL) of parents/caregivers in a southern Brazilian city before introduction of the pneumococcal vaccine in the National Immunization Program.
The study was conducted among privately insured children 0–5 years of age registered in a single private medical center in Paraná, Brazil, during September 2008–March 2010. Children who had no signs or symptoms of AOM or upper respiratory tract infection at the time of enrollment and whose parents/guardians provided written informed consent were followed for 12 months. The study was approved by a local independent ethics committee (Comitê de Ética em Pesquisa em Seres Humanos do Hospital de Clínicas da Universidade Federal do Paraná) and conducted according to the International Conference on Harmonization Guidelines for Good Clinical Practice, principles of Declaration of Helsinki.
Demographic data, medical history and pneumococcal vaccination history were collected from children's records. During the time of this study, Prevnar (7-valent PCV [PCV-7], Pfizer/Wyeth, USA) was available in private vaccination clinics.5 We classified children as vaccinated if, either they received at least two doses of pneumococcal conjugate vaccine (PCV) or received only one dose after one year of age at any time prior to onset of AOM episode, and unvaccinated if they received only one dose of a conjugate pneumococcal vaccine during the first year of life or unvaccinated (no dose received) at any time prior to onset of AOM episode.
AOM episodes diagnosed by a pediatrician and AOM-related procedures, such as tympanocentesis, adenectomy, transtympanic aerator tube insertion were recorded during a 12-month follow-up period. Parents/guardians were contacted every two months to solicit information on any respiratory or AOM related symptom lasting >48h, and visits to emergency rooms or other health care providers. A suspected AOM episode was defined as any AOM-related symptom reported only by parents, such as ear pain, discharge or tugging with one of the following symptoms: increased temperature, runny nose, sore-throat, cold-like symptoms, conjunctivitis, decrease in appetite, vomiting, diarrhea, trouble sleeping, irritability, or apathy. A probable episode was defined as an AOM episode diagnosed by a physician and documented in the medical chart, regardless of any documentation of symptoms. A probable AOM episode was confirmed when the visual appearance of the tympanic membrane was recorded (i.e. redness, bulging, loss of light reflex, presence of acute middle-ear effusion, as shown by otoscopy or tympanometry) and at least two of the following signs or symptoms were present: ear pain (otalgia), ear discharge, hearing loss, lethargy, irritability, anorexia, vomiting, diarrhea, fever (axillary temperature ≥38.0°C, rectal temperature ≥38.5°C) or analgesic/antipyretic therapy preceding fever. A new AOM episode was defined as an AOM episode after a 30-day symptom-free interval since the resolution of the previous AOM episode. AOM treatment failure was considered as persistence of AOM symptoms after 48–72h of treatment with appropriate antibiotics.
To assess the impact of AOM episodes on the QoL of parents/caregiver, parents were asked to complete a questionnaire within 14 days after the child was diagnosed with AOM. The questionnaire was based on the parental QoL questionnaire for recurrent ears, nose and throat (ENT) infections in their children (PAR-ENT-QoL),6 which was first adapted to be specific for AOM by a review panel from another study7 and then translated to Portuguese.
To estimated AOM incidence and 95% confidence interval (CI), overall, by age group, and pneumococcal vaccination status, we used the following formula:
where n is the total number subjects in the study cohort or subgroup; ¿i is the number of AOM episodes for subject i; δi is the number of days of the surveillance period for subject i.Among 2083 children assessed for participation, 1136 (55%) were enrolled in the study. Among 1074 (95%) children who completed the 12-month follow-up, the median age was 32 months (range: 0–71), 52.3% were males, 94.7% were white, 72.6% attended school or childcare center, 97.5% had history of being breastfed, and 32.2% had at least one more child ≤5 years in the household. For pneumococcal vaccination status, 69.2% (743/1074) were classified as vaccinated, 28.6% (307/1074) remained unvaccinated, the vaccination status was unknown for 2% (22/1074), and the date of onset of AOM was missing for two children who went on to become vaccinated during the follow-up.
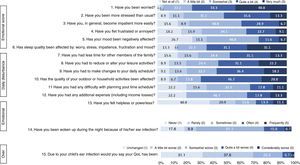
A total of 90 children experienced 99 AOM episodes, resulting in 133 health care center visits. Of the 99 AOM episodes recorded, seven (7%) were suspected and 92 (93%) were classified as probable, among which 45 (49%) were confirmed. Only one child had recurrent AOM episodes (i.e. at least three AOM episodes within six months). The most common clinical symptoms and signs of probable and confirmed AOM episodes were fever (67.4% and 86.7%, respectively), ear pain/otalgia (39.1% and 48.9%), redness of tympanic membrane (87.0% and 91.1%), bulging of tympanic membrane (50.0% and 55.6%), presence of acute middle-ear effusion (47.8% and 46.7%) and loss of light reflex (32.6% and 44.4%). Antibiotics were prescribed in 78.3% of probable AOM episodes and treatment failure was reported in 4.0%. Complications were reported for 4.3% AOM epsiodes, including persistent AOM, suppurative AOM, local abscess, and dysfunction of eustachian tube. Children were referred to consultation with otorhinolaryngologist in 7.6% of probable AOM episodes, and 2.2% had undergone any AOM-related procedure. None of the AOM episodes resulted in hospitalizations. The distribution of the QoL scores for emotional, daily disturbance and overall impact is described in Fig. 1. The highest scores, ≥3 on a scale of 1–5, were observed for worry (95.5%), impact on outdoor activities (84.5%), and sleep (84.4%). Overall, 68.9% of parents reported worsening (score 2–5) of their overall QoL, with 31.1% reporting somewhat considerable worsening (score 3–5).
The incidence of probable AOM was highest among 2-year old children (Table 1). The incidence of AOM in children ≤2 and 3–5 years of age was 105.5/1000 person-years (95% CI: 78.3–139.0) and 63.6/1000 person-years (95% CI: 43.2–90.3), respectively. The incidence of probable AOM among vaccinated and unvaccinated children was 86.3/1000 person-years (95% CI: 65.5–111.5) and 117.1/1000 person-years (95% CI: 80.1–165.3), respectively. The incidence of confirmed AOM among vaccinated and unvaccinated children was 49.1/1000 person-years (95% CI: 33.8–68.9) and 43.9/1000 person-years (95% CI: 22.7–76.7), respectively.
Incidence of probable AOM by age.
| Age (years) | Number of children | Total number of AOM episodes | Incidence (per 1000 person-years) | 95% CI | |
|---|---|---|---|---|---|
| LL | UL | ||||
| 0 to <1 | 208 | 10 | 89.7 | 43.0 | 165.0 |
| 1 to <2 | 185 | 18 | 101.9 | 60.4 | 161.0 |
| 2 to <3 | 198 | 22 | 118.3 | 74.1 | 179.1 |
| 3 to <4 | 173 | 16 | 86.2 | 49.3 | 140.0 |
| 4 to <5 | 166 | 10 | 63.0 | 30.2 | 115.8 |
| 5 to <6 | 144 | 5 | 35.0 | 11.4 | 81.8 |
AOM, acute otitis media; 95% CI, 95% confidence interval; LL, lower limit; UL, upper limit.
Note: Of the 92 probable AOM episodes, age at the time of the episode was missing for 11 subjects that were excluded from this analysis.
Our study provides estimates of AOM incidence among children ≤5 years of age attending a private medical center in Southern Brazil, and impact on QoL of parents/caregivers. We observed a much lower AOM incidence compared to that reported in European countries based on similar ascertainment methods and case definitions.8 Differences in health care seeking behavior, general health, and socio-economic status, in combination with true differences in AOM incidence in different populations may explain the variability in the results. Our study included a selected population of children with higher socio-economic status and access to private health care and vaccines. Consistent with this, the majority of AOM episodes was diagnosed by a pediatrician (93%), indicating that parents often sought health care and nearly 60–70% of our participants had received pneumococcal vaccination prior to the introduction of the vaccine in the national childhood schedule. A small number of children were referred to consultation with otorhinolaryngologist and none had undergone tympanocentesis, which is probably related to the small number of children with recurrent AOM or complications, such that the majority of AOM episodes is likely handled in pediatric offices and treated with oral antibiotics. Nonetheless, the majority of parents reported worsening of their overall QoL, which was similar to the findings in Europe.7
Because the number of AOM episodes were small, our study lacked statistical power to detect a significant difference in the incidence of probable AOM among vaccinated compared to unvaccinated children. However, a decrease in AOM incidence due to PCV vaccination has been reported in studies published previously.9,10 PCV-10 was introduced in the National Immunization Program in Brazil in April 2010, targeting all children <2 years of age.11 In 2011, the PCV-10 schedule included three doses plus one booster dose for children <1 year of age.12 Since then, high vaccination coverages have been attained. In 2014, PCV-10 coverage was 92.9% at country level.12 Studies assessing AOM incidence and etiology are needed to assess the impact of PCV-10 vaccination in Brazil. The incidence of probable AOM among unvaccinated children in our study may be useful as baseline data to assess impact of pneumococcal vaccine introduction in the Brazilian National Immunization Program in April 2010.
TrademarkPrevnar is a trade mark of Pfizer/Wyeth, USA.
FundingThis study was funded by GlaxoSmithKline Biologicals SA, Belgium (etrack number 112209). GlaxoSmithKline Biologicals SA was involved in all stages of study conduct and analysis and paid all costs associated with developing and publishing the manuscript.
Conflicts of interestRaghavendra Devadiga and Eduardo Ortega Barria are employed by the GSK group of companies. Tatiana M. Lanzieri, D. Fermin Arguello and Nervo Sanchez are former employees of the GSK group of companies Clóvis Arns da Cunha, Rejane B. Cunha have no conflicts of interest to declare.
The authors would like to acknowledge Lais Freitas (employed by Shift Gestão de Serviços on behalf of GSK), Rodrigo DeAntonio and all employees of GSK for their contribution to the study and the development of the paper. Acknowledgment is also made to Devayani Kolhe (employed by GSK) for the statistical analysis and development of the study report. The authors would also like to thank Cristina Frassetto and Eduardo Oliveira from GSK for their invaluable help with study set up. Uta Gomes (independent publication manager ginkgosolutions Ltd on behalf of GSK) and Gregory Collet (Business and Decision on behalf of GSK) for publication management and Varshini S (employed by GSK) and Kavi Littlewood (Littlewood Writing Solution on behalf of GSK) for writing support.