The dengue fever is a major public health problem in the world. In Brazil, in 2015, there were 1,534,932 cases, being 20,320 cases of severe form, and 811 deaths related to this disease. The distribution of Aedes aegypti, the vector, is extensive. Recently, Zika and Chikungunya viruses had arisen, sharing the same vector as dengue and became a huge public health issue. Without specific treatment, it is urgently required as an effective vector control. This article is focused on reviewing vector control strategies, their effectiveness, viability and economical impact. Among all, the Sterile Insect Technique is highlighted as the best option to be adopted in Brazil, once it is largely effectively used in the USA and Mexico for plagues related to agribusiness.
Dengue fever is a major public health problem in the world. The World Health Organization (WHO) estimates that 2/5 of the world's population are at risk of having the disease.1 It is the most common disease caused by an arbovirus.2 The distribution of Aedes aegypti, the vector, is widespread, along with the simultaneous circulation of four serotypes of the virus (DENV1, DENV2, DENV3, and DENV4).1,3
Associated with this problem, once they have the same vector, Zika and Chikungunya are on the rise. Since 2007, 55 countries from America, Asia, Africa, and Oceania have detected local transmission of Zyka virus. However, the first outbreak occurred in 2015 affecting almost 1.5 million people in Brazil, with 80% asymptomatic cases. Since then, it has been reported in 31 countries and American territories. Recently, a severe association between Zyka virus and microcephalia, retina lesions, and Guillian-Barrè syndrome have been reported.4 The recent outbreaks in the South America, Central America, and the Caribbeans represent the arbovirus most severe episode in the East hemisphere on the last 20 years.
Chikungunya emerged in 2013, and leaves a trail of joint symptoms. Notably, there are no specific treatments for these arboviruses. In this severe scenario, effective vector control is crucial, specially A. aegypti and Aedes albopictus, highly invasive species.5
In 2015, between January and November, 1.5 million Dengue cases was identified in Brazil, according to the Ministry of Health. In the same period, 17,146 Chikungunya suspect cases was identified, 6726 being confirmed later. Furthermore, Zyka was detected in 18 Brazilian states, with 739 suspected cases of microcephaly till 21st of November, 2015.6
In 2016, 1,399,480 probable Dengue cases were registered around the country, between 3rd of January and 9th of July. Additional 499,317 cases were dropped later. Regarding Zyka, 14,739 were registered as probable, 6903 being confirmed later by clinical and epidemiological criteria or laboratory testing. In relation to Chikungunya, it was 169,656 probable cases.7
Economic impactThe real economic impact of Dengue is not well documented. It is estimated as an average per patient of US 541 dollars and 1394 dollars, respectively, for outpatient and inpatient treatment. The mean disease duration is 11.9 days for outpatients and 11 days for inpatients. Among inpatients, students lost 5.6 school days and adults 9.9 working days due to this condition. This disease imposes substantial costs either on the health sector and general economy.8
The total estimated cost was 468 million dollars a year, in a study performed between 2009 and 2013. Adjusting for the unreported cases, the cost jumps to 1212 million dollars a year.9
The incidence, mortality, and morbidity of Dengue are underestimated. “Global Burden of Diseases Study, 2013” report estimated an average of 9221 deaths, caused by this arbovirus, a year, between 1990 and 2013, being 8277 in 1990 and peaking 11,302 in 2010. It resulted in a total of 576,900 years of life lost by premature death due to dengue fever in 2013 alone. The incidence considerably raised between 1990 and 2013, with the number of cases doubling each decade. Considering acute, mild and severe cases, and post-dengue chronic fatigue there will be about 566,000 lived years with incapacity. Considering fatal and non-fatal outcomes together, dengue was responsible for 1.14 million years lost due to incapacity, in 2013. It is believed that these numbers were even higher.2
In Brazil, the incidence in 2014 was 555,462 cases. The potentially severe clinical presentation accounted for 8975 cases and 453 deaths. In 2015, there were 1,534,932 cases, being 20,320 severe and 811 deaths.2,10 If laboratory testing were routinely performed on the suspect cases, there would certainly be even more diagnosed patients.11
Regarding Zyka and Chikungunya, the studies on incidence, mortality, morbidity, and sequelae rates of mid- and long-term are at early stages. In microcephaly cases due to Zika, it is estimated that the social and economical costs will be huge and long lasting.
The A. aegypti control and dengue prevention in São Paulo, Brazil, in 2005, had an estimated cost of 1.99 dollars per person. It was considered: human resources, uniforms, field material, individual protection material, pulverizing equipments, strategic supply (insecticide and larvicide), and vehicles. It should be added to the costs of laboratory testing, educational information, and press material.12 According to the Brazilian Ministry of Health, 2010–2014, the federal government spent around R$ 4.2 billion in preventive measures and Dengue treatment. During that period, the expenditure with the disease raised 48% – from R$ 613.4 million in 2010 to R$ 911.8 million in 2014.13
The Dengue vector and other virusesThe Dengue vector in Brazil, in urban areas is the A. aegypti.14 It is also the Yellow Fever, the Zika virus, and the Chikungunya vector. It prefers places with higher human concentration. Species from Africa, belong to the phylum Arthropoda, class Insecta, order Diptera, family Culicidae, genus Aedes. The reproduction and dispersion stages occur when the mosquito is adult. During the nuptial flight, the winged adult, female and male mate once. A single insemination is enough to fertilize all the eggs. They can travel up to 300m, and the pregnant female can fly up to 3km. They feed on plant syrup, but the female need blood protein to mature their eggs.15 Other species of the Aedes genus have been detected in Brazil.16
Vector control methodsThe Aedes mosquito is a crescent public health concern, and its control or eradication is urgent. The available or in development control methods can be divided into five categories: 1 – environmental breeding sites control; 2 – mechanical traps; 3 – insect fertility reduction technique; 4 – insecticides; and 5 – transgenic insect.17
Reduction of vector breeding sites in the environmentIt is the control of the vector breeding sites in standing water. Permanent actions decrease breeding focus. Education and population's participation are necessary. It is recommended continuing preventive measures and visits to all risk spots in order to fight the vector in early stages.1
Mechanical trapsThere are different types of traps, which imprison the insects, the eggs, and the larvae.
Insect fertility reduction techniquesThere are two biological strategies of vector control: Wolbachia related technique and sterile insect production by radiation technique.
On the first strategy, it was proposed the infection of Aedes mosquitoes with Wolbachia endosymbiotic, which inhibits the viral replication and dissemination. On the 14th day of infection, the Wolbachia completely blocks the Dengue transmission in at least 37.5%. These results highlight the potential usefulness of Wolbachia based strategies to protect the population from Dengue fever.18
On the second strategy, the males produced in laboratory are exposed to low radiation and sterilized, while keeping the copulation capacity. The insect made with impaired fertility is released into the environment to mate with wild insects and produce sterile eggs, eliminating then the next generation. In a study, the mating capabilities were compared, i.e. insemination ability, sterilizing ability, and mating competitiveness of irradiated male (35Gy) and the Wolbachia based strategy, with males of different ages. There was no significant difference on the insemination ability between irradiated and infected males.19
Chemical products to eliminate the insects or larvaeConsists on the insecticide use, with different pesticide application methods, for winged vector stage elimination. In order to prevent stings, it is used as repellents.
Genetic engineeringIt is based on the production of transgenic insects, which carry a dominant lethal allele capable of killing subsequent Aedes generations.20 This strategy was defended by a joint-venture formed by Oxitec and Moscamed. The Oxitec is a subsidiary company of Intrexon, biotechnology multinational listed on the stockmarket in New York. Moscamed is a Brazilian non-profit institution. The partnership has a modification insect factory. Mosquitoes designated OX513A are males and have received two genes. The first one is an activation system built from a synthetic DNA of fused Escherichia coli and herpes virus. The second is a marine coral gene from Discosoma, which works as a fluorescent marker so that the mosquitoes have different luminosity in comparison to wild ones, making possible to detect its presence by special light exposure. The first gene has the objective of shortening the insect's life and lead to that species termination in a region. This occurs as the modified males mate with wild females of the environment, producing descendents that will not survive till adult stage due to the lethal gene.21
DiscussionDengue, Zika and Chikungunya are relevant public health issues. Considering the morbimortality rate, the costs and the social-economic impacts, the control and extinction of these diseases would bring immeasurable gains for the nation.
There are three main options to solve the problems caused by these viruses: eliminate the virus, eliminate the vectors, or immunize people. The first option is not feasible as first approach.
Immunizing the population using an effective vaccine without adverse effects would be a great intervention. At the moment, Dengue vaccine is on field tests, but there is no vaccine for Zika or Chikungunya on the horizon.
Eliminate these viruses’ vectors would also solve the problem, and there are options in this line of action. The reduction of breeding sites in the environment, with elimination of the standing water reservoirs and larvicide use, demand permanent actions. In a similar way, the use of insecticide to control the adult mosquito. Education and population participation are necessary.1 In a tropical country, the climate and the rain conducive to proliferation of insects are a difficult obstacle. This line of action has been the backbone of the Brazilian Government Dengue and other arboviruses control strategies, with international organizations support, such as WHO. Nonetheless, Dengue fever still has frequent intense outbreaks. Recently, a Brazilian Minister of Health came to public to say that the current methods used for vector control have been ineffective. In addition, there is the arousal of Chikungunya and Zika.
The techniques of insect's next generation elimination consist on the induction of sterile eggs production, or young sick insects with shorter life cycle. These are attractive options because they would ultimately eliminate the vectors, irrespective of educational measures, climate, and does not use pesticides.
Regarding the use of transgenic insect technique, in 2012 an attempt was made in Juazeiro and Jacobina cities, state of Bahia, Brazil, by releasing those insects in the suburbs. An 80% reduction on the A. aegypti's larvae population was reported, together with 95% reduction of adult winged mosquitoes. These results were described in an independent review published on “PLoS Neglected Tropical Diseases”, in July 2015. Other independent studies on the efficacy of the OX513A to be conducted by Professor Margareth Capurro, University of Sao Paulo, could not be concluded due to budget cuts. However, preliminary data suggested the modified insect's efficacy. The test in Jacobina, Bahia showed a 79% reduction of the insect population. Despite the success news spreading by the media, the test did not leave good impression among locals, and there were important demonstrations against it due to striking raise in Dengue fever cases in the city. Even taking into account that the modified insects were not released in the whole urban area, it was expected a reduction of clinical cases. The issue was largely explored at BBC news.21
The CNTBio, organ of the Science, Technology and Innovation Ministry, released the commercial use of the transgenic mosquito in spite of concerns expressed by civil organizations and the news of Jacobina. Since April 2015, Piracicaba city, Sao Paulo, was the first to adopt the transgenics as part of the Dengue control program. As of March 2015, the Public Ministry recommended the suspension of mosquito release at the city, under the argument that the efficacy had not been proved and alluded to the Jacobina episode. The decision was revised one month later and the mosquitoes were release. It is estimated that 23 million mosquitoes had already been released in Cecap and Eldorado suburbs. The Piracicaba press informed that it was too early to evaluate the results because there was no baseline for comparison. Local data revealed that 60% of captured larvae had inherited the modified gene. However, statistics showed that there were 934 Dengue cases in 2014, while there was already 3668 confirmed cases till 7th of December, 2015.21
In 2016, Piracicaba registered 142 confirmed Dengue cases and 714 notifications till 19th of February, according to local Health Secretary. Also, there were eight suspected cases of Chikungunya. The number confirmed cases of Dengue would be 238% higher than 2015, if compared to the data published by the government at the beginning of 2014. The notification rate in early 2016 was also 99% higher than 2015.22
Regarding sterile eggs production technique, there are two main techniques: Wolbachia based technique and the radiation sterilized insects. The produced insect with impaired fertility is released on the environment to mate wild insects and generate sterile eggs, eliminating the next generation.
On the first strategy, it was proposed the infection of Aedes mosquitoes with Wolbachia endosymbiotic, which inhibits the viral replication and dissemination. On the 14th day of infection, the Wolbachia completely blocks the Dengue transmission in at least 37.5%. These results highlight the potential usefulness of Wolbachia based strategies to protect the population from Dengue fever.18
On the second strategy, the males produced in laboratory are exposed to low radiation and sterilized, however keeping the copulation capacity. The insect made with impaired fertility is released into the environment to mate with wild insects and produce sterile eggs, eliminating then the next generation. In a study, the mating capabilities were compared, i.e. insemination ability, sterilizing ability, and mating competitiveness of irradiated males 35Gy and the Wolbachia based strategy, with males of different ages. There was no significant difference on the insemination ability between irradiated and infected males.19
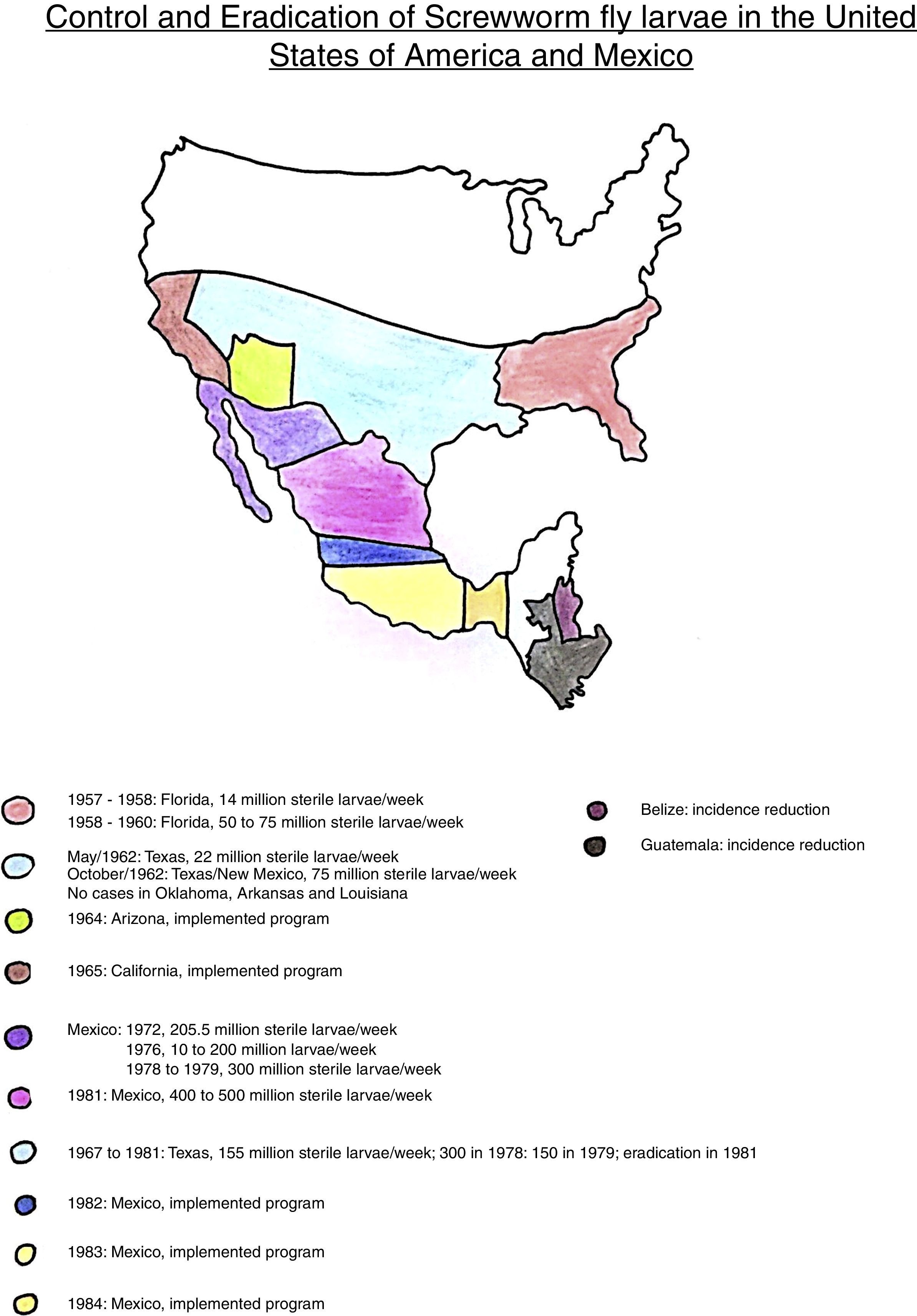
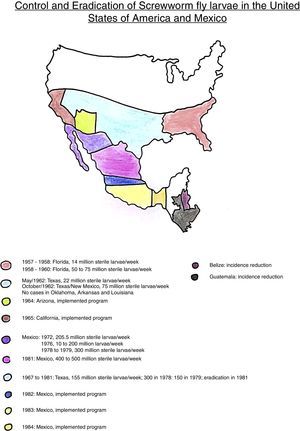
The sterile insect technique (SIT) was designed and created by the American Entomologist E.F. Knipling as an alternative for vector control or even eradication of the screwworm fly, Cochliomyia hominivorax, which used to plague the cattle farms in the USA, affecting the leather industry. The final result was a fantastic success. They got rid of the bug. Afterwards, the project expanded to the south, involving Mexico, Central America, and Colombia. This last country, considered the plague border, has an alert system: whenever the insect reappears coming from South America, new release of sterile insects should be implemented in order to control it.23
The eradication cronology of the screwworm fly in the Americas, using SIT, is shown on the adapted map (Fig. 1).
Map adapted from James E. Novy.26
The economic impact was significant while controlling this leather plague using SIT. Before eradication, there was a high cost to farmers, who had to spend on trained cavalry for periodic herd inspection, facilities, annual loss of 10% of the cattle herd (Texas), wound treatment, pesticide use, leather's damage and depreciation. In 1957, before eradication, the berne plague was responsible for a livestock industry loss of U$ 20 million a year, on the Southeast.24 On the same way, in 1960, the cattle loss was estimated in more than U$ 100 million annually. An economic study, in 1972, estimated that the reinfection on the South would cost to the livestock industry about U$ 372 million.19
The cost of eradication programs, in 1976, was approximately U$ 15 million. Considering the potential losses of U$ 372 million, there was a significant economic return. Also, it was estimated that the economic benefit for the cattle farmers as result of plague control, between 1962 and 1976, was higher than U$ 1 billion.19
The SIT uses ionizing radiation to induce random chromossomic rearrangement, leading to sterile males. It was the first sterile-male system developed. The currently most employed method for insect and mite sterilization is through ionizing radiation, derived from Co60 and Cs137 radioisotopes, accelerator (operates under 10MeV) generated electrons, and X-ray generated by electron beam with energy under 5MeV. The neutrons are more effective than X-ray and gamma radiation for insect sterilization. However, neutron can easily induce radioactivity and, due to immobility and difficult availability of nuclear reactors (source of neutrons), its use would not be feasible in most of the SIT projects. According to Food and Agriculture Organization of the United Nations (FAO), one sterile insect is defined as one insect submitted to a proper treatment that makes it unable to produce viable offspring.
The concept of Plague Integrated Management (PIM) gained further popularity after the 70s, when the pesticide use suffered restrictions. At the International Plant Protection Convention (IPPC) was established that the SIT is a biological control strategy, being incorporated to eradication programs or control over large areas, whose planned actions have to be implemented by a management coordination program, acting regionally, beyond the limits of rural or urban propriety.
With the SIT development, there was also the amplification and development of new science areas such as: biology and nutrition of insects, insect mass production, molecular biology, insect behavior, and control over large areas models.25
Regarding screwworm control, in the 40s, months after weekly release of SIT screwworms on the Curacao Island, the plague was eradicated. Today, lots of countries have national SIT programs with bioindustries for C. capitata creation (USA, Mexico, Guatemala, Argentina, Chile, Peru, Portugal, Tunisia, Thailand, and South Africa), some species of genus Anastrepha (Mexico and USA) and Bactrocera (USA, Japan, Malaysia) for control and/or eradication. The use of this technique expansion have proved success in protecting fruit-growing areas against Mediterranean fly infestation.23
Despite originally proposed to eradicate livestock and agriculture plagues, the technique awaken the possibility of controlling human diseases’ vectors due to its undeniable success.1 If it shows efficacy on controlling human diseases’ vectors, there would translate in exceptional gains. Some researchers have published the theoretical basis for the SIT use on the combat against Aedes.
The SIT has been used in lots of programs with success against different agricultural plagues as well as insects of medical importance such as Tse-Tse mosquitoes. Phase I studies have shown its potential in controlling the mosquito population. The sterile males have to meet a standardized quality control established by the International Agency of Atomic Energy, in which the insects have to be capable of flying, attract females, mate, and transferring semen despite being infertile.
Bellini et al. conducted a pilot experiment using SIT in five clinical trials to develop a methodology to suppress the populations of A. albopictus, in three small cities in north Italy, between 2005 and 2009. Created male insects, sexed by a sieving technique that allows recovery of about 26–29% of males, were exposed to gamma radiation and immediately released in the environment. The results showed that sterile male released at a rate of 896–1590 insects/ha induced a significant sterile level on the wild population of insects. They have estimated that the objective of keeping 81% of sterile eggs leads to insect suppression. Immigration of pregnant females was not the main concern over the small villages, where the tests were carried out.23
The SIT has been studied on the A. albopictus control. This vector has global expansion, and the absence of vaccines against the majority of arboviruses transmitted by these vectors has stimulated the development of control strategies with SIT in order to control disease transmission by controlling natural vectors.
Proposal of vector control by sterile insect techniqueDengue fever endemic situation has to be faced along with its expensive preventive control strategies and therapeutic demand. In addition, the currently used methods have proved ineffective. Therefore, SIT would theorically be the best option. It is urgent the use of an effective method, specially with the Zika and Chikungunya virus on the rise, whose stratospheric costs have not yet been estimated. SIT has the advantage of being a method that does not pollute the environment, leads to eradication, and does not depend on sanitary education of the population. It is effective by itself.
It is necessary to attract academic zeal, and the enthusiasm of politicians and administrative authorities, notwithstanding this technique has no “lobby” and would not generate profit unlike pesticide and medicines. It is the only method that has proved efficacious in large scale vector control of other insect species and has already pilot studies with promising results with the Aedes genus. When planning the use of SIT, it is necessary to understand Aedes’ reproduction behavior on the environment:
- a)
Male agglomeration;
- b)
Sexual pheromone release;
- c)
Copulation call;
- d)
Female approximation;
- e)
Copulation beginning (the female can accept or not the male);
- f)
Copulation ending;
- g)
Duration of copulation;
- h)
In case spermatheca is not filled, the female can seek for other males.
After copulation, the females begin the search for an oviposition site.19 The SIT implementation depends, exclusively, on the academic enthusiasm defended by specialists. At the moment, it is not supported by any private company on the stockmarket, and has no political influence to support it.
Brazil already has some experience with SIT use. A biofactory, sterile insect producer, named Moscamed was built in Juazeiro, Bahia, in 2005. It is mainly focused on plague control (C. capitata) in the agribusiness. Its location is strategic once San Francisco valley is responsible for more than 95% mango and grapes exportation of the country. In addition, this other fruit flies (Anastrepha oblique and A. fraterculus) present in the region are considered plagues with quaternary importance in some countries like USA, Japan, Europe, and Asia. Therefore, it is necessity to maintain a low prevalence of fruit flies’ population on the region, i.e. Fly/Trap/Day rate lower than 1.0 (FTD<1.0).16
In September 2015, the Agriculture, Livestock and Supply Ministry launched the Fly Fruit National Combat Program aiming to installing and maintaining risk mitigation systems, certification and eradication programs. In this case, the program aims to implement actions to eradicate Ceratitis capitata and Anastrepha fraterculus in fruit production and exportation zones, looking forward to providing healthy fruits and ensuring quaternary safety to the importers.
The SIT is still an underexplored strategy option in Brazil. However, the success of this technique all over the world stimulates the increase of official management and eradication programs in agroecosystems and rural/urban areas.
Theorically, a project of this scale aiming to eradicate Aedes from our environment, would have to meet the following requirements:
- -
Logic, science and practice well-founded project;
- -
Sponsorship of a strong political and administrative group;
- -
Viability of manufacturing the sterile mosquito;
- -
Viability in keeping the sterile vector alive, for a sufficient period till releasing it on the environment;
- -
Sterile vector's ability to copulate with the wild insects;
- -
Sterile eggs as a result of the reproduction process;
- -
Create methodology for evaluating the results;
- -
Discover the needed frequency and duration of insect release into the environment to control or extinguish the wild vectors;
- -
Plan preparation of new release of the sterile mosquitoes if the vector reappears after some time;
- -
Whole operation cost analysis.
In addition to the above items, it is highly needed to convince the relevant authority. A task force has to be built by Brazilian and international specialists with proved experience on the issue, to study, plan, and make the project viable followed by a pilot implementation. After demonstration of viability, the project would then be expanded to the rest of the country.
ConclusionDengue remains a major public health problem in the world. The WHO estimates that 2.5 billion people were affected in 2013. Dengue is the world's more frequent arbovirus. In Brazil, the incidence in 2014 was of 555,462 cases. Associated with this problem, once they share the same vector, there is Zika and Chikungunya on the rise. The potentially severe clinical presentation occurred in 8975 cases with 453 deaths. In 2015, there were 1,534,932 cases, 20,320 of whom were severe with 811 deaths. The real economic impact of the disease is poorly documented, and it is estimated an average 541 dollars and 1394 dollars per patient, respectively, for outpatient and inpatient treatment. The total estimated cost was 468 million dollars a year, in a study conducted between 2009 and 2013. Adjusting the unreported results, the cost jumps to 1212 million dollars a year. There has been an effort toward Dengue and arboviruses control, however more intense outbreaks happen frequently. Recently, the Brazilian Minister of Health came to public saying that the methods currently used for vector control have proved ineffective. In this context, a firm action to resolve the problem is warranted and vector control through SIT is undoubtedly the most effective strategy, irrespective of the population's sanitary education.
Conflicts of interestThe authors declare no conflicts of interest.