This cross-sectional study assessed the immunization status of human immune deficiency virus (HIV)-infected patients receiving care at an outpatient clinic in Brazil. The sociodemographic characteristics, CD4 count and HIV viral load of 281 out of 612 adult outpatients were analyzed. A total of 331 patients were excluded because of no availability of vaccination cards. Chi-square or Fisher's exact test were used. Immunization coverage was higher for diphtheria/tetanus (59.79%) and hepatitis B (56.7%), and lowest for hepatitis A (6.8%) and for meningococcal group C (6%). Only 11.74% of the patients had received the influenza virus vaccine yearly since their HIV-infection diagnosis. No vaccination against influenza (p<0.034) or hepatitis B (p<0.029) were associated with CD4 counts <500cells/mL; no vaccination against flu or pneumococcus were associated with detectable HIV viral load (p<0.049 and p<0.002, respectively). Immunization coverage is still very low among HIV-infected adults in this setting despite recommendations and high infection-related mortality.
The new potent therapies introduced in mid-1990s significantly reduced Acquired Immunodeficiency Syndrome (AIDS)-related mortality. Brazil was a pioneer in implementing universal access to modern antiretroviral therapy within the country's public healthcare system. In 2014, more than 400,000 individuals were estimated to be on these drugs.1 Nevertheless, likely due to late diagnosis (26% of patients were still initiating treatment with a CD4T lymphocyte count below 200cells/mL in October 2014)1 or because of patients’ difficulty in complying with treatment, many deaths from AIDS were still reported in this setting. The latest epidemiological bulletin published by the Ministry of Health reported that mortality resulting from AIDS declined by only 6% in Brazil over the past ten years, falling from 6.1 deaths per 100,000 inhabitants in 2004 to 5.7 per 100,000 in 2013.1
The main cause of mortality in HIV-infected patients between 2009 and 2013 in the Brazilian state of Espírito Santo were infectious and parasitic diseases.2
The National Immunization Program has specific recommendations for HIV-infected patients.3 These recommendations are also emphasized by the National AIDS Program, in its clinical protocol and therapeutic guidelines for the management of HIV infection in adults.4 Nevertheless, there has been no assessment of the effective implementation of these immunization protocols in the daily routine of services dedicated to the care of AIDS patients in Brazil.
The main objective of the present study was to assess immunization coverage in a referral center for HIV-infected patients in accordance with the recommendations published by the Ministry of Health.
Materials and methodsA cross-sectional, observational study was conducted to assess the immunization status of HIV-infected individuals receiving care at an infectious diseases outpatient clinic in a large philanthropic hospital. The study was conducted between January 2015 and February 2016.
The sample size was calculated taking into account the proportion of individuals immunized against various different diseases to be evaluated. Considering the population of approximately 1000 HIV-infected patients being followed up at the referral center, for an expected prevalence of immunized individuals of 40%, a sample error of 5%, and significance level of 5%, the minimum required sample size was defined as 270 patients. Allowing for an exclusion rate of 50% (patients losing or forgetting to bring in their immunization record card), the minimum number of patients required to screen for admission to the study was established as 540. The sample consisted of HIV-infected individuals over 18 years of age who were selected randomly while consulting with physicians at the infectious diseases outpatient clinic.
The Institution internal review board approved the study protocol under reference CAAE 42811014.4.0000.5065. All the participants signed an informed consent form.
The patients’ records were reviewed and a previously validated questionnaire was used to collect data on age, sex, mode of HIV transmission, antiretroviral drugs in use, date of the HIV-infection diagnosis, last CD4 cell count and HIV viral load, and hepatitis B surface antibody (anti-HBs) status.
The latest guidelines from the Ministry of Health indicate the following vaccines for HIV-infected patients: diphtheria/tetanus, pneumococcal, influenza, hepatitis B, hepatitis A, and meningococcal group C.3 Patients were considered appropriately immunized if they had had four double doses of the hepatitis B vaccine, three doses of the diphtheria/tetanus vaccine (with the latest within 10 years), annual influenza vaccines from the date of HIV diagnosis until the date of the interview, two doses of the hepatitis A vaccine, two doses of the 23-valent polysaccharide pneumococcal vaccine, with a minimum interval of five years between the two doses (in the case of patients diagnosed more than five years previously), and two doses of the meningococcal group C conjugate vaccine. For analysis of the hepatitis B vaccine, the patient's status of hepatitis B surface antibody was checked to define their antibody status for the disease. The yellow fever vaccine was not included in this investigation, since yellow fever was not considered endemic in this state at the time this study was done. Other live attenuated vaccines (measles, mumps, rubella, and varicella), particularly recommended in the case of pediatric patients, albeit contingent on CD4 levels, were also not assessed, since children were not included in this study sample. For the same reason, immunization coverage with respect to the human papillomavirus (HPV) vaccine, which was introduced during the study period for females aged 9–26 years, was not a focus of this study.5 Chi-square or Fisher's exact test (in the case of expected values<5) was used to verify associations between categorical variables. The standardized residuals were analyzed and compared with a critical value of 1.96, and significance was established at 5%.
All the patients whose immunization was incomplete were referred to the Special Immunobiology Referral Center (CRIES) to rectify the situation.
ResultsInitially, 612 patients were screened. Of these, 331 individuals failed to bring their vaccination cards to any of the consultations they attended during the study period despite being repeatedly requested to do so. These patients were excluded from the study and the final sample consisted of the remaining 281 patients, 58 of whom had no vaccination card because they had never been vaccinated.
Most of the patients were male (52.31%). The mode of HIV transmission was heterosexual exposure in 69.39% of cases. Only 2.5% of the patients had yet to commence treatment. Overall, 80.1% of the patients had an HIV-1 viral load below detectable limits (<50copies/mL) and 63.3% had a CD4 lymphocyte count>500cells/mL (Table 1).
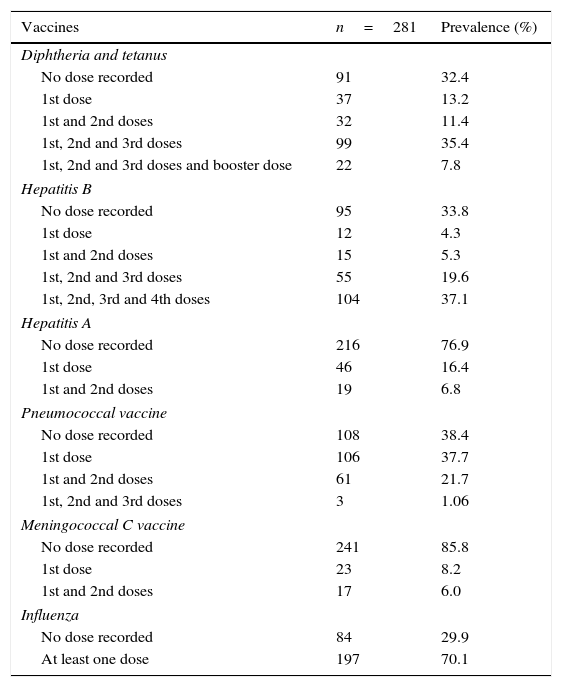
Total number of doses of each vaccine used by the HIV-infected patients. Vitória, Espírito Santo, Brazil.
| Vaccines | n=281 | Prevalence (%) |
|---|---|---|
| Diphtheria and tetanus | ||
| No dose recorded | 91 | 32.4 |
| 1st dose | 37 | 13.2 |
| 1st and 2nd doses | 32 | 11.4 |
| 1st, 2nd and 3rd doses | 99 | 35.4 |
| 1st, 2nd and 3rd doses and booster dose | 22 | 7.8 |
| Hepatitis B | ||
| No dose recorded | 95 | 33.8 |
| 1st dose | 12 | 4.3 |
| 1st and 2nd doses | 15 | 5.3 |
| 1st, 2nd and 3rd doses | 55 | 19.6 |
| 1st, 2nd, 3rd and 4th doses | 104 | 37.1 |
| Hepatitis A | ||
| No dose recorded | 216 | 76.9 |
| 1st dose | 46 | 16.4 |
| 1st and 2nd doses | 19 | 6.8 |
| Pneumococcal vaccine | ||
| No dose recorded | 108 | 38.4 |
| 1st dose | 106 | 37.7 |
| 1st and 2nd doses | 61 | 21.7 |
| 1st, 2nd and 3rd doses | 3 | 1.06 |
| Meningococcal C vaccine | ||
| No dose recorded | 241 | 85.8 |
| 1st dose | 23 | 8.2 |
| 1st and 2nd doses | 17 | 6.0 |
| Influenza | ||
| No dose recorded | 84 | 29.9 |
| At least one dose | 197 | 70.1 |
In this study sample, 223 patients (79.4%) had a vaccination card and presented it during one of the consultations. The best immunization coverage (59.79%) was against diphtheria/tetanus. Around 7.45% of the individuals had failed to complete the required immunization schedule for this vaccine and in 32.74% of cases this information was absent in their vaccination cards.
Of the patients assessed, 159 (56.7%) had received at least three doses of the hepatitis B vaccine, while 37.1% of these had received the regimen of four doses, as recommended for HIV-infected patients.1 Vaccination was incomplete in 9.6% of cases and 33.8% of the patients had not been vaccinated at all against hepatitis B (Table 1). Only 50.17% of the participants had been tested for hepatitis B surface antibody (anti-HBs) previously. No statistically significant association was found between the number of doses received of the vaccine against hepatitis B and anti-HBs positivity.
Immunization against hepatitis A was complete in only 6.8% of the patients, while 76.9% had not received any dose of this vaccine. Only 11.74% of the study patients had received the annual influenza vaccine correctly ever since the date of their HIV diagnosis. On the other hand, 30.25% had never been vaccinated against influenza and 58% had not completed the full immunization calendar, with 56.93% of these individuals having received less than half of the recommended number of doses.
Sixty-five patients (23.2%) had received two doses of the 23-valent polysaccharide pneumococcal vaccine, with immunization being incomplete in 38.1% of the participants and another 38.8% never having been vaccinated at all (Table 1). Only 6% of the patients had received complete immunization with the meningococcal C vaccine, while 85.8% had not received any dose of the vaccine.
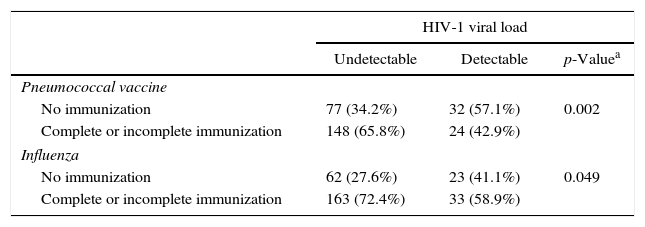
A statistically significant association was found between detectable HIV viral load and vaccine coverage against pneumococcal or influenza (p<0.05). From the residual value of the tests, the number of individuals who had not been immunized with these two vaccines was significantly greater (adjusted residual>1.96) in the group with detectable HIV viral load. The association between influenza and hepatitis B coverage with CD4 lymphocyte count was also statistically significant. The number of individuals who had not been vaccinated against hepatitis B or influenza was significantly greater (adjusted residual>1.96) in the group with CD4 lymphocyte count<500cells/mL (Table 2).
Association between vaccine coverage with CD4 count and HIV viral load.
| HIV-1 viral load | |||
|---|---|---|---|
| Undetectable | Detectable | p-Valuea | |
| Pneumococcal vaccine | |||
| No immunization | 77 (34.2%) | 32 (57.1%) | 0.002 |
| Complete or incomplete immunization | 148 (65.8%) | 24 (42.9%) | |
| Influenza | |||
| No immunization | 62 (27.6%) | 23 (41.1%) | 0.049 |
| Complete or incomplete immunization | 163 (72.4%) | 33 (58.9%) | |
| CD4 (cells/mL) | |||
|---|---|---|---|
| <500 | ≥500 | ||
| Hepatitis B | |||
| No immunization | 41 (41.8%) | 52 (28.9%) | 0.029 |
| Complete or incomplete immunization | 57 (58.2%) | 128 (71.1%) | |
| Influenza | |||
| No immunization | 37 (37.8%) | 46 (25.6%) | 0.034 |
| Complete or incomplete immunization | 61 (62.2%) | 134 (74.4%) | |
The present study was specifically designed to assess the effective use of inactivated vaccines involving killed organisms, which make up the basis of the vaccines recommended for HIV-infected adults in accordance with the National Immunization Program.3 Traditionally, coverage in Brazil has been adequate with respect to child immunization. Immunization of immunosuppressed adults such as HIV-infected patients is centralized in special immunobiological referral centers (CRIES). In the state of Espírito Santo, such a center is located in a pediatric hospital in the state capital city.
The present findings show that immunization coverage in this study sample was disappointingly low. The main bias in this study was that we have only accessed patients that had brought their vaccination cards after at least two repeated requests (less than half of the patients screened). If those patients excluded for not having vaccination cards are assumed to be non-vaccinated, the coverage rates showed here would be overestimated by at least 50%. What are the reasons behind such exceptionally low coverage? At the beginning of the AIDS epidemic it was found that vaccination could transitorily increase HIV viral load.6,7 Nevertheless, more recent studies conducted after the introduction of the more potent antiretroviral drugs currently used (highly active antiretroviral therapy [HAART]), showed neither significant changes in viral load following vaccination8–10 nor appearance of resistance mutations,11 although some fluctuations occur justifying current recommendations for not measuring HIV viral load in the weeks following vaccination.4
A recent review of the causes of mortality from AIDS in the state of Espirito Santo between 2009 and 2013 showed that the main causes of death were infectious and parasitic diseases.2 The TEMPRANO study, conducted in Sub-Saharan Africa and recently published, also highlighted the risk of mortality from infectious diseases, particularly invasive bacterial diseases and tuberculosis, risks that are reduced when antiretroviral therapy is initiated early.12 Vaccines constitute one of the most effective means of preventing infectious diseases. Expanding immunization coverage is considered one of the most cost-effective ways of promoting health and one of the principal factors responsible for the increase in life expectancy in the general population over the past ten years.13
Of all the vaccines evaluated in this study, the vaccine against diphtheria/tetanus showed the best rate of complete coverage (59.79%), which could be explained by the wide availability of this vaccine in the health clinics in general not just at the CRIES. In addition, it is formally recommended in cases of accidents involving risk. Although it has been known for quite some time that the risk of hepatitis B is greater among HIV-infected individuals,14 only 56.7% of the participants in the present study had been immunized against hepatitis B. This vaccine is also available in general healthcare clinics. Although the most commonly recommended regimen of four double doses is part of the treatment protocol in Brazil, only 37.1% of the patients had been completely immunized. No statistically significant association was found between immunization coverage against hepatitis B and positivity for anti-HBs, probably because there was no synchronization between vaccination and anti-HBs testing, since assessing response to immunization in HIV-infected patients is not routinely done.
Around 70.1% of patients had received at least one dose of influenza vaccine; however, only 11.74% had received the annual dose every year since the date of their HIV-infection diagnosis. Considering that the government launches an annual campaign to promote influenza vaccine, the compliance of the public is good, and the vaccine is available in all healthcare units, the use of the influenza vaccine in this population is far from satisfactory. After all, the clinical efficacy of the influenza vaccine in HIV-infected patients has already been confirmed in a meta-analysis.15 Overall, 38% of the patients in the present study had not received the 23-valent polysaccharide anti-pneumococcal vaccine; even though all cause pneumonia is responsible for 20.25% of deaths among HIV-infected patients in this state,2 and the efficacy of this vaccine has already been confirmed in other studies conducted with similar populations.16,17
In this study, the lowest immunization coverages were against hepatitis A (6.8%) and meningococcal group C (6%). This may have been due to these vaccines being recommended for HIV-infected patients as recently as 2014,3 and probably being less incorporated into the routine care provided by attending physicians. In addition, these vaccines are only available at the CRIES.
Two other studies conducted in Brazil, one in São Paulo published in 200818 and one in Fortaleza published in 2016,19 also analyzed the immunization status of HIV-infected adults. Those studies included 144 and 99 patients, respectively, and also reported very low rates of coverage. Martins et al. studied vaccination coverage against hepatitis B in around 300 HIV-infected patients in the south of Brazil and found fairly similar rates to those reported here (57.4% compared to 56.7% in the present study).20
An important limitation of the present study was the fact that the vaccines were not available in the outpatient clinic in which the HIV-infected patients were seen. If this service had been offered, it could have served as a stimulus for the patients to bring their vaccination cards to all the consultations to enable their vaccination status to be brought up to date in real time.
Centralizing vaccines for immunosuppressed individuals at the CRIES, although facilitating public service logistics, may hamper patient compliance, since they need to take another day off work in addition to that already required to attend the consultation. Moreover, the extra trip for vaccination involves paying for more public transportation. During the study period, there was a shortage of certain vaccines at the CRIES, particularly the hepatitis B, hepatitis A, and the 23-valent pneumococcal vaccines, which may also have contributed to the poor coverage detected in this study. The main reasons disclosed by the patients for not having received their vaccines were no availability in local units close to their home address, or no availability even at the CRIES when they had enough time to look for these vaccines at the only reference center existing in Vitoria, Espirito Santo.
We observed an association between CD4 cell count<500/mL and detectable levels of HIV viral load in plasma with non-vaccination against flu, pneumococcal, or hepatitis B, the most commonly prescribed vaccines to HIV-infected patients. We suggest that the patients in whom compliance with antiretroviral therapy was poorest or whose response to treatment was poorer were less likely to follow recommendations regarding immunization. However, guidance on immunization should be given and constitute a formal part of the consultations provided by doctors and other health professionals responsible for the treatment of these patients. In our belief, it is a matter of replicating the immunization framework practiced by pediatricians and incorporating it into the practice of physicians who treat adults, making the prescription of vaccines part of every medical consultation involving HIV-infected patients.
ConclusionsImmunization coverage remains very low in HIV-infected adults in this setting despite the official recommendations from the health authorities in the country and the high infection-related mortality that persists in this population. The availability of those vaccines for immunosuppressed individuals only at reference centers distant from where patients live and work contributes to low coverage. Vaccines should be available as antiretroviral drugs where patients are cared.
Conflicts of interestThe authors declare no conflicts of interest.