The objective of this study was to investigate bacterial resistance trends, infection sites and the relationship between resistance and admittance to the intensive care unit (ICU). A total of 53,316 bacteria identified between 1999 and 2008 were evaluated. Multidrug resistance was characterized when gram-negative bacilli (GNB) presented resistance to two or more classes of antibiotics. Gram-positive cocci (CPC) were assessed for resistance to penicillin, oxacillin and vancomycin. GNB were the most common (66.1%) isolate. There was a 3.7-fold overall increase in multidrug resistant GNB over the study period; Acinetobacter baumanii and Staphylococcus aureus were the most prevalent. Highest increases were recorded for Klebsiella pneumoniae (14.6-fold) and enterococci (73-fold). The resistance rates for GNB and GPC were 36% and 51.7%, respectively. Most multidrug resistant GNB and GPC were recovered from ICU patients (p-value<0.001). Vancomycin-resistant enterococci were isolated during this decade with an increase of 18.7% by 2008. These data confirm the worldwide trend in multidrug bacterial resistance.
The introduction of antibiotics in the 1930s allowed for a change in hospital care and the development of progressively more sophisticated procedures. However, in the 1950s and 1960s there was a pandemic of nosocomial infections caused by Staphylococcus aureus. In the 1970s, gram-negative bacilli (GNB) resistant to certain antibiotics became the most common hospital-acquired pathogens. In the 1980s oxacillin-resistant staphylococci became a major concern and the importance of enterococci increased. During the 1990s, antibiotic-resistant organisms became even more important in the hospitals environment, particularly in intensive care units (ICUs), which were at the epicenter of Multidrug Resistant bacteria (MDR). This further undermined the survival of patients and allowed the spread of resistant pathogens to other hospital units, to other healthcare services and to the community at large.
Resistance to antibiotics is considered a major threat to health by the World Health Organization. The European Community reported 25,000 deaths related to infection by MDR at a cost of 1.5 billion Euros.1,2 Therefore, the development of new antibiotics has become of major global concern.
According to the literature, variations in resistance rates and mechanisms underlying the characteristics of each geographical region and each institution make knowledge of local epidemiology essential. The aim of this study was to report the main multidrug resistant agents, trends, origins/types of specimen and the relationship between the nature of infection and admittance to the ICU.
This study was conducted in a teaching hospital affiliated to a Medical School; 80% of patients are under the auspices of the National Health Service. It is a 716-bed (89 in ICUs) general hospital. Highly complex procedures are carried out including organ transplants (bone marrow, liver, kidney, heart, etc.) and infant heart surgery.
All hospital GNB and gram-positive cocci (GPC) were isolated during the period from January 1st 1999 to December 31st 2008 and were identified at the Microbiology Laboratory database of the institution. Isolates were not distinguished from colonization or infection. To avoid duplication of results, only the first sample from each source in a given month was considered, except for catheters related samples. Isolates whose antibiogram yielded resistant or intermediate results were deemed resistant. GNB were defined as MDR when there were resistance to two or more distinct classes of antibiotics; all antibiotics of a particular class should have yielded intermediate or resistant results. The following classes of antibiotics were tested: aminoglycosides, quinolones, beta-lactams (cephalosporins, beta-lactamase inhibitors, carbapenems) and polymyxin B. Beta lactam antibiotics were evaluated individually.
Resistance was defined for specific GPC: oxacillin-resistant Staphylococcus aureus, oxacillin-resistant coagulase-negative staphylococci, penicillin-resistant streptococci, and vancomycin-resistant enterococci were characterized on the grounds of antibiogram results. The laboratory routinely uses the Kirby–Bauer antibiotic test. Variables that were evaluated included microbial species, origin/type of specimen and the hospital unit where the patient was hospitalized (ICU and non-ICU).
During the study period, 53,316 nosocomial bacteria were isolated, 35,250 of which (66.1%) were GNB. The 12 most common GNB, totaling 29,824 (84.6%), were selected to evaluate resistance; the remainder were of no apparent microbiological or epidemiological interest according to the more prevalent nosocomial agents. Overall 10,732 (36%) were characterized as MDR.
GPC accounted for 18,066 (33.9%) of hospital-acquired bacteria; 17,575 (97.3%) were selected to evaluate resistance as the remaining GPC had either incomplete antibiograms or lacked apparent microbiological or epidemiological interest. Of these, 9086 (51.7%) were characterized as resistant.
Of the MDR GNB (10,732), the most frequent five bacteria (9416 – 87.7%) were chosen for analysis. These included Acinetobacter baumanii (3410 – 31.8%), Pseudomonas aeruginosa (2700 – 25.1%), Klebsiella pneumoniae (2173 – 20.2%), Escherichia coli (577 – 5.4%) and Citrobacter freundii (556 – 5.2%). Resistant GPC were evaluated in four groups, as follows: S. aureus (8345 – 47.5%), coagulase-negative staphylococci (4986 – 28.4%), enterococci (2571 – 15.6%) and streptococci (1673 – 9.5%).
MDR GNB and resistant GPC isolates were recovered from respiratory tract (25.4% and 11.3%), urinary tract (30% and 5%), blood, and catheters (23.8% and 50.5%), fluids and non-respiratory tract secretions (17.4% and 27.4%) and other specimens (3.4% and 5.8%).
A total of 6314 MDR GNB were isolated from ICU patients and 3102 from non-ICU patients (p-value<0.001). Moreover, 2438 resistant GPC and 4552 sensitive GPC were isolated from ICU patients compared to 6051 and 4534, respectively from non-ICU patients (p-value<0.001).
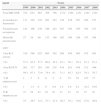
In 1999, 332 MDR GNB were identified and while 1221 MDR GNB were isolated in 2008. Details of the changes in numbers and percentages of the isolates are shown in Table 1.
Total multidrug resistant gram-negative bacilli (GNB) and most common species and percentage of resistant gram-positive cocci (GPC) from January 1st 1999 to December 31st 2008 in a tertiary university hospital.
| Agent | Years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | |
| Total MR GNB | 332 | 652 | 663 | 768 | 798 | 1174 | 1364 | 1251 | 1193 | 1221 |
| Acinetobacter baumannii | 111 | 228 | 242 | 261 | 222 | 338 | 538 | 507 | 426 | 537 |
| Pseudomonas aeruginosa | 142 | 108 | 238 | 246 | 242 | 283 | 382 | 385 | 302 | 211 |
| klebsiella pneumoniae | 27 | 59 | 90 | 135 | 198 | 365 | 304 | 248 | 353 | 394 |
| GPC | ||||||||||
| Oxa-R S. aureus | 318 | 546 | 625 | 605 | 382 | 268 | 459 | 465 | 475 | 363 |
| (%) | 57.3 | 62.5 | 57.3 | 60.9 | 45.2 | 47.1 | 58.1 | 55.4 | 51.3 | 51.3 |
| Oxa-R ECN | 261 | 317 | 261 | 189 | 238 | 418 | 621 | 458 | 512 | 446 |
| (%) | 59.3 | 67.3 | 73.9 | 74.4 | 63 | 71.1 | 85.7 | 82.5 | 78.2 | 78.7 |
| V-R enterococcus | 1 | 2 | 0 | 0 | 2 | 4 | 19 | 24 | 147 | 73 |
| (%) | 1 | 1.3 | 0 | 0 | 0.9 | 1.4 | 5.8 | 8.1 | 34.2 | 19.6 |
| P-R streptococcus | 37 | 54 | 83 | 83 | 61 | 51 | 47 | 22 | 27 | 32 |
| (%) | 33 | 26.7 | 32.1 | 37.7 | 27.4 | 26.3 | 35.1 | 19.6 | 27.6 | 26.9 |
MR, multidrug resistant; GNB, gram-negative bacilli; GPC, gram-positive cocci; Oxa-R, oxacillin-resistant; P-R, penicillin-resistant; V-R, vancomycin-resistant.
It is important to remember that some of these isolates may be due to contamination of the collected specimens or represent colonization or pre-existent infections of patients. The quantification and correlation of these isolates were not beyond the scope of the current study.
During the study period the frequency of nosocomial GNB was twofold higher than that of GPC which highlights the importance of these agents. The total number of cases of MDR GNB per year (Table 1) increased 3.7-fold during one decade. The three most common MDR GNB, Acinetobacter baumannii, Pseudomonas aeruginosa and klebsiella pneumoniae, accounted for 77.1% of all GNB with 4.8-fold, 1.5-fold, and 14.6-fold increases, respectively over the study period; this underlines the global trend in multiresistance.3,4 The frequencies of the isolates related to the different wards and types of specimens were similar. The number of MDR GNB isolates was statistically higher in the ICU compared to other units of the hospital, a fact that has been widely reported in the literature.5
S. aureus was the most prevalent resistant GPC accounting for almost half (47.5%) of the total number of cases, as was reported by The Surveillance Network (TSN), a network of 300 laboratories in the USA.6 The number of cases of both S. aureus and streptococci remained relatively stable over the decade. However, there was a 1.7-fold increase in coagulase-negative staphylococci during the study period. Table 1 shows the dramatic increase in resistant enterococci during the decade of the study, with a 73-fold increase and a peak of 147 isolates. According to the records the first vancomycin-resistant enterococci isolated in this hospital was in urine in August 1998, 10 years after the first vancomycin-resistant enterococci had been identified in the world and two years after the first isolate identified in Brazil.7 For samples originating from the respiratory and urinary tracts there was a smaller proportion of resistant GPC compared to MDR GNB. The opposite was seen for blood and catheters. The high rate of resistant GPC isolates (50.5%) from blood and catheters was mainly related to coagulase-negative staphylococci, a fact that has already been reported in the literature.8 Resistant GPC are equally found in the ICU and in other units of the hospital (4552/4616), in contrast to antibiotic-sensitive GPC (ICU 2348 and non-ICU 6051) for which there was a significant difference (p-value<0.001) underscoring the importance of these agents in the ICU.
Although the overall rate of resistant GPC (52.8%) was higher than the MDR GNB (36%), the major concern today is the lack of therapeutic options for the treatment of the latter.1 This work did not analyze the occurrence of resistance to specific antibiotics according to GNB species. The results of this work illustrate that S. aureus and streptococci resistance remained relatively stable over the decade (Table 1). However, coagulase-negative staphylococci resistance increased by 19.3% during the period, besides presenting a higher level of resistance than S. aureus. There was a real emergency with respect to resistance of enterococci: starting at 1%, the annual resistance increased to 34.2% and then dropped back to 19.6% in 2008 giving a total increase over the period of 18.7%.
In conclusion, over the decade evaluated in this study there was a high prevalence of MDR GNB, in particular Acinetobacter baumannii and klebsiella pneumoniae. There was also a gradual increase in the multidrug resistant GNB. S. aureus became the most prevalent GPC and vancomycin-resistant enterococci have emerged during this decade. These two classes of bacteria were significantly more frequently isolated in the ICU. For this institution, the emergence of vancomycin-resistant enterococci was the main concern in the evaluated decade. Measures to prevent and control the dissemination of infectious agents and optimization of antibiotic use must be considered priorities. Future studies specifically evaluating molecular microbiology of MDR GNB species and enterococci, the site of infections, analysis of hospital units, the evolution of antibiotic resistant MDR GNB and consumption of antimicrobials are needed. Finally, this synopsis confirms the trend in multidrug resistance in a single center over the past decade as a reflection of a phenomenon that may occur worldwide.
Conflict of interestThe authors declare no conflict of interest.
The authors acknowledge the assistance of Mrs Delzi Vinha Nunes de Góngora and Adília Maria Pires Sciarra.