To analyze the effectiveness and the safety of Sofosbuvir-based regimens to treat patients with chronic hepatitis C virus (HCV) infection and chronic kidney disease (CKD).
MethodsA retrospective, observational study in patients with chronic HCV infection and CKD treated with Sofosbuvir-based regimens was performed. Liver fibrosis, comorbidities, HCV genotype and sustained virological resposnse (SVR) at 12th week post-treatment were evaluated. Kidney function was accessed by serum creatinine and glomerular filtration rate (GFR). The assumed level of significance was 5 %.
ResultsThirty-five patients were treated. The mean age was 52.1±10.9 years, 19 (54.3 %) were women, 32 (91.4 %) were already kidney transplanted and 3 (8.6 %) were on hemodialysis. The SVR by intention to treat was 88.6 %. The mean GFR was 65.8±28.6 and 63.7±28.3ml/min pre- and post-treatment respectively (p>0.05). Treatment was interrupted in 1 (2.85 %) patient due to anemia and in 2 (5.7 %) due to loss of kidney function.
ConclusionSofosbuvir-based regimens are effective to treat HCV in patients with CKD. In patients with mild CKD this type of therapy seems to be safe.
The prevalence of hepatitis C virus (HCV) infection in patients with end-stage renal disease (ESRD) is clearly higher than the observed in the general population.1 In Brazil, 1.38 % of the population is infected with HCV,2 and in patients on dialysis, the prevalence of this infection ranges from 4.2%–8% depending on the renal replacement therapy center.3
HCV infection in patients with chronic kidney disease (CKD) is associated with a more rapid progression of liver disease, increased mortality due to complications of cirrhosis, loss of renal graft and glomerulonephritis, thus contributing to a worse prognosis in these patients.4 Despite the relationship between HCV and the progression of CKD, the vast majority of patients with CKD remained untreated, because they are historically a difficult to treat population due to the frequent adverse effects associated with medications used to treat HCV.5,6 In addition to the complexity of the treatment, the use of Interferon in renal transplant recipients is associated with a high risk of graft rejection (15%–100%) and has been contraindicated in this set.7
The advent of direct-acting antivirals (DAA) dramatically changed the treatment of HCV, including the special population of patients with CKD. In 2015, the use of Sofosbuvir (SOF) based regimens was approved in Brazil.8 Renal transplant patients and patients on hemodialysis have been treated with DAA since then, but the importance of individualizing the treatment due to the risk of loss of kidney function was also emphasized.
The objective of the present study was to analyze the effectiveness and the safety of Sofosbuvir-based regimens to treat patients with CKD.
Material and methodsA retrospective, observational study was performed in all cronically infected patients with HCV and CKD at various stages treated with SOF-based regimens at the Gastroenterology/Hepatology outpatient clinic of the Santa Casa Hospital, Porto Alegre, a tertiary reference hospital from southern Brazil, from June 2016 to March 2018.
The age, sex, liver fibrosis, comorbidities, HCV genotype and sustained virological response (SVR) at 12th week post-treatment were evaluated.
Kidney function was accessed by serum creatinine and GFR calculated using the Cockroff-Gault formula before, after treatment and 3 months after the end of treatment.5 Chronic kidney disease was classified at different stages according to criteria established by Kidney Disease Improving Global Outocomes (KDIGO).5 Kidney transplantation prior to treatment and the use of immunosuppressants were also registered.
Chronic HCV infection was defined as the persistence of HCV-RNA for a period of at least six months. Molecular diagnosis HCV were performed by polymerase chain reaction (PCR) - Real-time using the Extraction and Amplification Method: COBAS® AmpliPrep / COBAS® TaqMan® HCV Quantitative Test, v2.0 (Roche).
Patients were considered to have cirrhosis based on clinical, image, laboratory or histologic parameters (METAVIR score).9
Treatment with DAA was performed according to the Brazilian Public Health system (2015),8 which recommended that patients with CKD and kidney transplant patients should be treated with a non-interferon-alpha regimen and, if possible, without ribavirin.
In the statistical analysis, the quantitative variables were described by mean and standard deviation (if normal distribution) or median and interquartile range (if asymmetric distribution). The normality of the continuous variables was evaluated by the Shapiro-Wilk test. Categorical variables were described by absolute and relative frequencies. To compare means before and after treatment, the paired t-student test was applied. In case of asymmetry, the Wilcoxon test was used. For the categorical variables, the McNemar test was applied. To compare means between the three moments, the analysis of variance (ANOVA) for repeated measures was applied. In case of asymmetry or ordinal variables, the Friedman test was used. The association between numerical and ordinal variables was evaluated by the Spearman correlation coefficient. The significance level adopted was 5 % (p<0.05) and the analyzes were performed in the SPSS program version 21.0. The study was approved by the Research Ethics of local Committee (number 1838479).
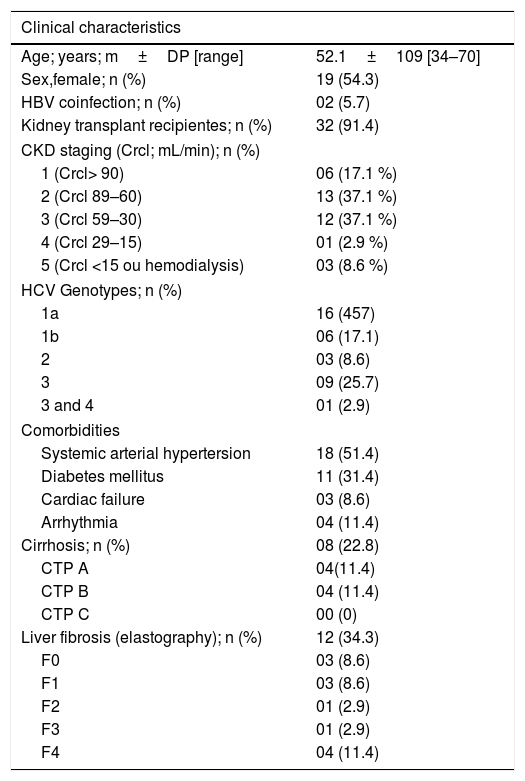
ResultsThirty-five patients were analyzed. There was a light predominance of females 19 (54.3 %), the mean age was 52.1±10.1 years (34–70 years), 32 (91.4 %) were previously submitted to renal transplants and 3 (8.6 %) patients were on hemodialysis (Table 1).
Clinical characteristics of patients with HCV and CKD (n=35).
| Clinical characteristics | |
|---|---|
| Age; years; m±DP [range] | 52.1±109 [34–70] |
| Sex,female; n (%) | 19 (54.3) |
| HBV coinfection; n (%) | 02 (5.7) |
| Kidney transplant recipientes; n (%) | 32 (91.4) |
| CKD staging (Crcl; mL/min); n (%) | |
| 1 (Crcl> 90) | 06 (17.1 %) |
| 2 (Crcl 89–60) | 13 (37.1 %) |
| 3 (Crcl 59–30) | 12 (37.1 %) |
| 4 (Crcl 29–15) | 01 (2.9 %) |
| 5 (Crcl <15 ou hemodialysis) | 03 (8.6 %) |
| HCV Genotypes; n (%) | |
| 1a | 16 (457) |
| 1b | 06 (17.1) |
| 2 | 03 (8.6) |
| 3 | 09 (25.7) |
| 3 and 4 | 01 (2.9) |
| Comorbidities | |
| Systemic arterial hypertersion | 18 (51.4) |
| Diabetes mellitus | 11 (31.4) |
| Cardiac failure | 03 (8.6) |
| Arrhythmia | 04 (11.4) |
| Cirrhosis; n (%) | 08 (22.8) |
| CTP A | 04(11.4) |
| CTP B | 04 (11.4) |
| CTP C | 00 (0) |
| Liver fibrosis (elastography); n (%) | 12 (34.3) |
| F0 | 03 (8.6) |
| F1 | 03 (8.6) |
| F2 | 01 (2.9) |
| F3 | 01 (2.9) |
| F4 | 04 (11.4) |
CDK=chronic kidney disease; CTP=Child Turcotte- Pugh escore; CrCl: Creatinine clearance; HBV: Hepatitis B virus; HCV: Hepatitis C vírus.
Considering the stages of CKD, 16 (45.7 %) were stage 3 or worse. Regarding the etiology of kidney failure, 24 (68.6 %) had no diagnosis, and systemic lupus erythomatous was found in 3 (8.6 %) patients. Glomerulonephritis focal segmental scleroderma (GESF), diabetes mellitus (DM), systemic arterial hypertension, polycystic kidney disease, Alport syndrome, chronic pyelonephritis and Bor syndrome were responsible for the etiology in a few patients (8; 22.8 %). HCV genotype 1 was the most prevalent (22; 62.8 %) (Table 1).
Coinfection with hepatitis B virus (HBV) was found in 2 patients (5.7 %). There was observed the reactivation of HBV in one of these, immediatly post-treatment of HCV. This was a renal transplant, genotype 1 patient, using Tenofovir since 2012, with undetectable HBV viral load pre-treatment. The patient presented SVR after HCV treatment, and after HBV reactivation. Entecavir was associated with Tenofovir.
In the evaluation of liver fibrosis, no patient underwent liver biopsy. Transient hepatic elastography (THE) was performed in 12 (34.2 %) patients, showing absence or mild/significant fibrosis (F0-F1-F2) in 7 (58.3 %) and advanced fibrosis (F3-F4) in 5 (41.7 %) patients. Cirrhosis was diagnosed by THE in 4 (11.4 %) patients, and by clinical presentation (portal hypertension with ascites and esophageal varices) in 4 (11.4 %). Among the 8 cirrhotics, 5 (14.2 %) were Child-Turcotte-Pugh (CTP) A and 3 (8.6 %) CTP B (Table 1).
The prevalent comorbidities were systemic hypertension in 18 (51.4) patients, diabetes mellitus in 11 (31.4 %), heart failure in 3 (8.6 %) and cardiac arrhythmia in 4 (11.4 %) (Table1).
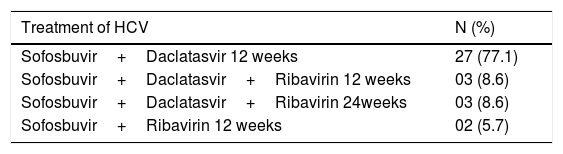
Of the 35 patients who underwent HCV treatment, 33 (94.2 %) were treated with Sofosbuvir and Daclatasvir, of whom 8 (24.2 %) received Ribavirin. The 2 (5.7 %) patients with HCV genotype 2 were treated with Sofosbuvir and Ribavirin (Table 2). Full dose of Sofosbuvir was used in 33 (94.2 %) patients, and half dose (1 tablet on alternate days) in 2 (5.7 %) patients.
Medications used in the treatment of patients with HCV and CKD (n=35).
| Treatment of HCV | N (%) |
|---|---|
| Sofosbuvir+Daclatasvir 12 weeks | 27 (77.1) |
| Sofosbuvir+Daclatasvir+Ribavirin 12 weeks | 03 (8.6) |
| Sofosbuvir+Daclatasvir+Ribavirin 24weeks | 03 (8.6) |
| Sofosbuvir+Ribavirin 12 weeks | 02 (5.7) |
HCV=hepatitis C virus.
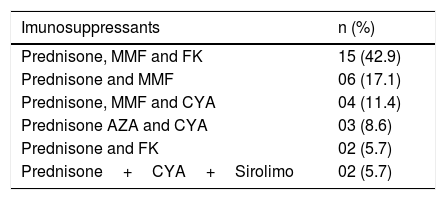
Thirty-two (91.4 %) patients were receiving immunosuppressants because they were kidney transplant recipients. Immunosuppressive regimens were not modified during HCV treatment. There was a predominance of Prednisone associated with Mycophenolate Mofetil and Tacrolimus in 15 (42.9 %) patients (Table 3).
Imunosuppressants in CKD submitted to renal transplantation (n=32).
| Imunosuppressants | n (%) |
|---|---|
| Prednisone, MMF and FK | 15 (42.9) |
| Prednisone and MMF | 06 (17.1) |
| Prednisone, MMF and CYA | 04 (11.4) |
| Prednisone AZA and CYA | 03 (8.6) |
| Prednisone and FK | 02 (5.7) |
| Prednisone+CYA+Sirolimo | 02 (5.7) |
AZA: Azathioprine; CYA: cyclosporin; FK: Tracolimus; MMF: Mycophenolate mofetil.
The treatment was suspended in 3 (8.6 %) patients, being 1 (2.8 %) due to anemia and 2 (5.8 %) due to a significant loss of kidney function. The patient who discontinued treatment for symptomatic anemia (hemoglobin drop) was cirrhotic on hemodialysis using Sofosbuvir, Daclatasvir and Ribavirin. The two patients worsening kidney function were cirrhotic Child-Turcotte-Pugh B, renal transplanted, in the advanced stages of CKD stage 3 (GFR 41mL/min) and stage 4 (GFR 27mL/min), worsening to GFR 29ml/min and 25mLmin, respectively.
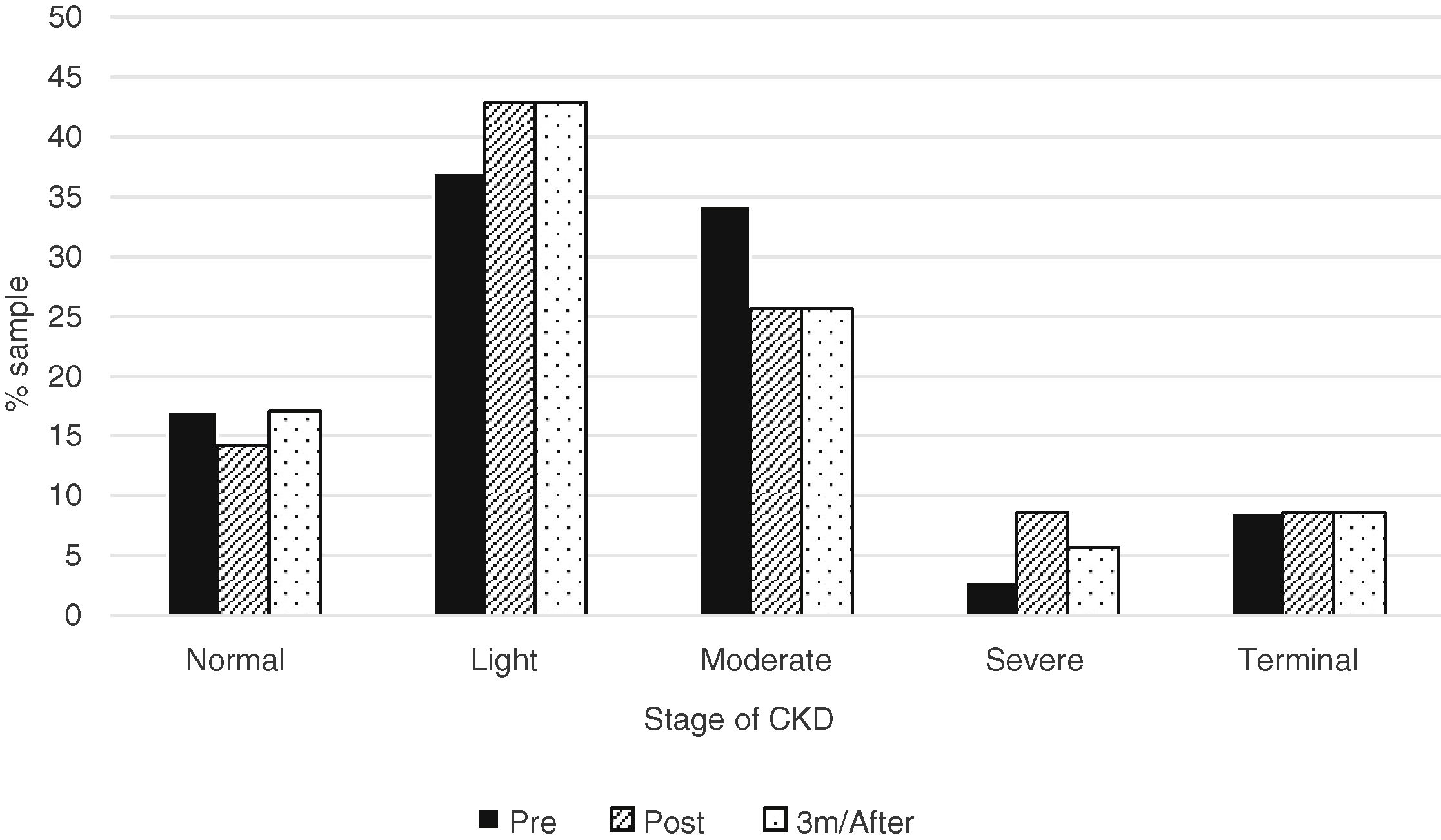
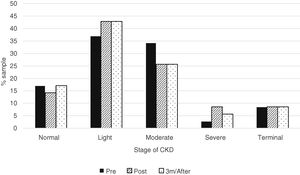
When the median creatinine and GFR were analyzed, there was no statistically significant difference before and after treatment with Sofosbuvir (Table 4). And also, there was no statistically significant difference when analyzed the CKD according to the different stages (Fig. 1).
Comparison of creatinine and glomerular filtration rate (GFR) pre, post and three months after treatment.
| Variables | Pre | Post | 3m after EOT | P |
|---|---|---|---|---|
| Creatinine (mg/dL); Median (P25–P75) | 1.27 (0.94–1.57) | 1.23 (0.92–1.70) | 1.28 (0.92–1.92) | 0.573a |
| GFR (mL/min); Median±SD | 65.8±28.6 | 63.7±28.3 | 64.4±29,8 | 0.241b |
M=months; treat=treatment; GFR=Glomerular filtration rate; SD=standard deviation; EOT=end of treatment.
SVR was evaluated in 32 patients who completed the treatment, and 31 (88.6 %) achieved SVR 12 in an intention to treat analysis; per protocol 96.9 %. Only 1 (2.8 %) presented HCV recurrence after treatment.
DiscussionIn the present study, a series of cases of patients with CKD and HCV were treated with Sofosbuvir-based regimens, successfully achieving SVR and without GFR decline. Most of the patients were renal transplant patients, non-cirrhotic patients with HCV genotype 1, and the most commonly used treatment regimen was the association of Sofosbuvir and Daclatasvir for 12 weeks.
Hepatitis C virus often has a negative impact on the survival of post-renal transplant patients due to the increased risk of graft loss due to rejection and disease infection, post-transplant diabetes, cancer and rapid progression of liver fibrosis.10
Although eradication of HCV is of paramount importance, renal transplant recipients have always represented a special population difficult to treat. Currently, the use of DAA has been an important step in the treatment of HCV infection due to rates of SVR ranging from 60 % to 100 % according to genotype and degree of hepatic dysfunction.11
Manys studies in the literature that evaluate the use of DAA in CKD presented with encouraging results. Desnoyer et al.12 observed that Sofosbuvir was well tolerated in 13 dialysis patients. However, SVR12 was 100 % in patients who used Sofosbuvir full dose and 60 % in those who received three times a week. Saxena et al., (n=19, being 5 on hemodialysis) observed, in the TARGET study, no difference in the SVR regardless of kidney function. However, patients with GFR<30ml/min experienced more anemia and worsened kidney function after using Sofosbuvir.13 Studies on kidney transplant recipients treated with DAAs stand out with 100 % SVR12.14–16 This study confirms that Sofosbuvir-based therapies are effective in patients with CKD. In this series of 35 cases, the SVR12 was reached in 31 (88.6 %) patients. Only one patient (2.8 %) developed HCV recurrence after treatment; he was genotype 1a, under hemodialysis, presented fibrosis F1 evaluated by THE and was treated with Sofosbuvir, Daclastavir and Ribavirin for 12 weeks.
Treatment of hepatitis C with new DAA may represent a risk for reactivation of hepatitis B virus in co-infected patients with HCV. A systematic review and meta-analysis found 17 studies involving 1621 patients being 242 with HBV and 1379 with resolved hepatitis B (HBsAg-negative and anti- HBc-positive). All were treated with different regimens containing DAA against HCV. The reacivation of HBV was observed in 24 % (IC95 % 19–30 %) in patients with chronic HBV infection and 1.4 % (0.8–2.4 %) in those with resolved HBV infection.17 In the present study, 2 (5.7 %) patients were HBsAg positive, and one reactivated HBV during use de DAA.
To know the drug interactions of DAA is of fundamental importance, particularly the interaction between protease inhibitors and calcineurin inhibitors or mammalian target of rapamycin (mTOR). Simeprevir, a second-generation antiviral that inhibits the protease coded by the HCV NS3/4A region, has potential drug interaction with Tacrolimus, Sirolimus and Everolimus.18
Sofosbuvir (polymerase inhibitor encoded by the HCV NS5B region) and Daclatasvir (HCV NS5A region-encoded polymerase inhibitor) have a low risk of interaction with immunosuppressants and have pangenotypic action. In this study, the vast majority of patients were renal transplant patients (n=33; 94.3 %).
All patients were treated with Sofosbuvir based regimes. Renal transplant patients were using immunosuppressants, 8 (25.0 %) used schedules with Ciclosporin, and 17 (48.5 %) with Tacrolimus. There was no need for more frequent control of the levels of immunosuppression due to the lack of drug interaction with these DAA. This study showed that treatment with Sofosbuvir was well tolerated in patients with mild CKD, although 3 (8.6 %) patients discontinued therapy.
A pontential limitation of the present study the fact that it is retrospective and included a small number of patients in more advanced stages of CKD.
In conclusion, this study suggests that tratament HCV in patients with CKD is efetive, and in patients with mild CKD this type of therapy seems to be safe.
Conflicts of interestThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no conflicts of interest.