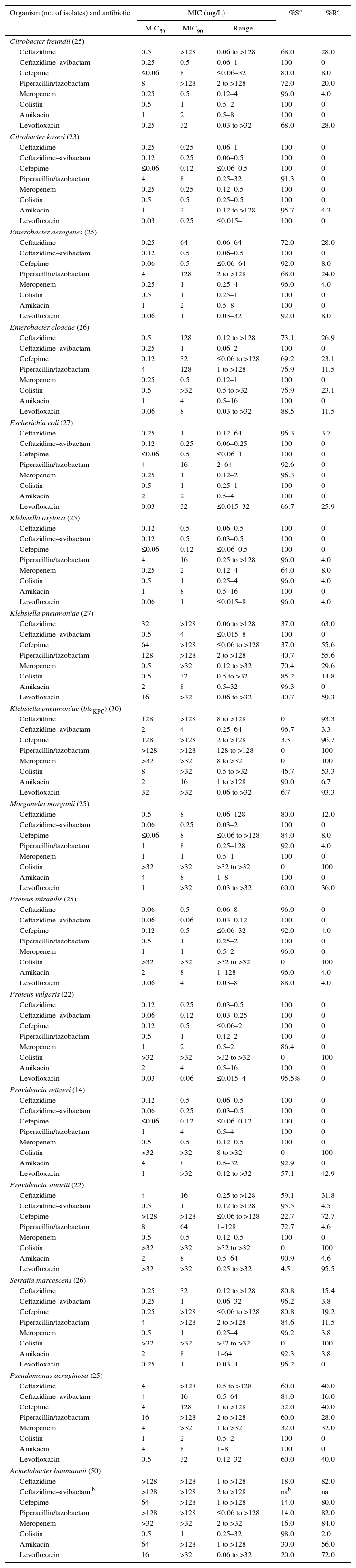
Avibactam is a new non-β-lactam β-lactamase inhibitor that restores the in vitro activity of ceftazidime against isolates of Enterobacteriaceae and Pseudomonas aeruginosa that harbor Ambler molecular class A, class C and some class D β-lactamases,1 but not those harboring metallo-β-lactamases.2 Ceftazidime–avibactam and comparator antibacterial agents were tested by reference broth microdilution against 417 non-repetitive Gram-negative bacilli (387 unselected, plus 30 selected blaKPC-positive, meropenem–nonsusceptible, Klebsiella pneumoniae) collected prospectively from medical centers at Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Brazil, in 2014 and 2015. Specimen sources were associated with bloodstream, respiratory, urinary, gynecological, intra-abdominal, wound, or skin and skin structure infections. Isolates defined as “non-selected” were collected without phenotypic pre-selection so that they represented those encountered clinically. Only one isolate per patient was included in the study. Minimum inhibitory concentrations (MICs), one per isolate, were determined by reference Clinical and Laboratory Standards Institute (CLSI) broth microdilution methods3 using frozen microtiter plates pre-loaded with antibiotic-containing growth medium. MICs of ceftazidime–avibactam were measured by varying the concentration of ceftazidime in twofold increments with avibactam at a fixed concentration of 4mg/L.3 Quality control (data not shown) was achieved according to CLSI guidelines3 using American Type Culture Collection (ATCC) isolates, Escherichia coli ATCC 25922, E. coli ATCC 35218, K. pneumoniae ATCC 700603, and P. aeruginosa ATCC 27853. The test results are summarized in Table 1.
In vitro activities of ceftazidime, ceftazidime–avibactam, and comparator antibiotics against Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii isolated from patients in São Paulo, Brazil, 2014–2015.
| Organism (no. of isolates) and antibiotic | MIC (mg/L) | %Sa | %Ra | ||
|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | |||
| Citrobacter freundii (25) | |||||
| Ceftazidime | 0.5 | >128 | 0.06 to >128 | 68.0 | 28.0 |
| Ceftazidime–avibactam | 0.25 | 0.5 | 0.06–1 | 100 | 0 |
| Cefepime | ≤0.06 | 8 | ≤0.06–32 | 80.0 | 8.0 |
| Piperacillin/tazobactam | 8 | >128 | 2 to >128 | 72.0 | 20.0 |
| Meropenem | 0.25 | 0.5 | 0.12–4 | 96.0 | 4.0 |
| Colistin | 0.5 | 1 | 0.5–2 | 100 | 0 |
| Amikacin | 1 | 2 | 0.5–8 | 100 | 0 |
| Levofloxacin | 0.25 | 32 | 0.03 to >32 | 68.0 | 28.0 |
| Citrobacter koseri (23) | |||||
| Ceftazidime | 0.25 | 0.25 | 0.06–1 | 100 | 0 |
| Ceftazidime–avibactam | 0.12 | 0.25 | 0.06–0.5 | 100 | 0 |
| Cefepime | ≤0.06 | 0.12 | ≤0.06–0.5 | 100 | 0 |
| Piperacillin/tazobactam | 4 | 8 | 0.25–32 | 91.3 | 0 |
| Meropenem | 0.25 | 0.25 | 0.12–0.5 | 100 | 0 |
| Colistin | 0.5 | 0.5 | 0.25–0.5 | 100 | 0 |
| Amikacin | 1 | 2 | 0.12 to >128 | 95.7 | 4.3 |
| Levofloxacin | 0.03 | 0.25 | ≤0.015–1 | 100 | 0 |
| Enterobacter aerogenes (25) | |||||
| Ceftazidime | 0.25 | 64 | 0.06–64 | 72.0 | 28.0 |
| Ceftazidime–avibactam | 0.12 | 0.5 | 0.06–0.5 | 100 | 0 |
| Cefepime | 0.06 | 0.5 | ≤0.06–64 | 92.0 | 8.0 |
| Piperacillin/tazobactam | 4 | 128 | 2 to >128 | 68.0 | 24.0 |
| Meropenem | 0.25 | 1 | 0.25–4 | 96.0 | 4.0 |
| Colistin | 0.5 | 1 | 0.25–1 | 100 | 0 |
| Amikacin | 1 | 2 | 0.5–8 | 100 | 0 |
| Levofloxacin | 0.06 | 1 | 0.03–32 | 92.0 | 8.0 |
| Enterobacter cloacae (26) | |||||
| Ceftazidime | 0.5 | 128 | 0.12 to >128 | 73.1 | 26.9 |
| Ceftazidime–avibactam | 0.25 | 1 | 0.06–2 | 100 | 0 |
| Cefepime | 0.12 | 32 | ≤0.06 to >128 | 69.2 | 23.1 |
| Piperacillin/tazobactam | 4 | 128 | 1 to >128 | 76.9 | 11.5 |
| Meropenem | 0.25 | 0.5 | 0.12–1 | 100 | 0 |
| Colistin | 0.5 | >32 | 0.5 to >32 | 76.9 | 23.1 |
| Amikacin | 1 | 4 | 0.5–16 | 100 | 0 |
| Levofloxacin | 0.06 | 8 | 0.03 to >32 | 88.5 | 11.5 |
| Escherichia coli (27) | |||||
| Ceftazidime | 0.25 | 1 | 0.12–64 | 96.3 | 3.7 |
| Ceftazidime–avibactam | 0.12 | 0.25 | 0.06–0.25 | 100 | 0 |
| Cefepime | ≤0.06 | 0.5 | ≤0.06–1 | 100 | 0 |
| Piperacillin/tazobactam | 4 | 16 | 2–64 | 92.6 | 0 |
| Meropenem | 0.25 | 1 | 0.12–2 | 96.3 | 0 |
| Colistin | 0.5 | 1 | 0.25–1 | 100 | 0 |
| Amikacin | 2 | 2 | 0.5–4 | 100 | 0 |
| Levofloxacin | 0.03 | 32 | ≤0.015–32 | 66.7 | 25.9 |
| Klebsiella oxytoca (25) | |||||
| Ceftazidime | 0.12 | 0.5 | 0.06–0.5 | 100 | 0 |
| Ceftazidime–avibactam | 0.12 | 0.5 | 0.03–0.5 | 100 | 0 |
| Cefepime | ≤0.06 | 0.12 | ≤0.06–0.5 | 100 | 0 |
| Piperacillin/tazobactam | 4 | 16 | 0.25 to >128 | 96.0 | 4.0 |
| Meropenem | 0.25 | 2 | 0.12–4 | 64.0 | 8.0 |
| Colistin | 0.5 | 1 | 0.25–4 | 96.0 | 4.0 |
| Amikacin | 1 | 8 | 0.5–16 | 100 | 0 |
| Levofloxacin | 0.06 | 1 | ≤0.015–8 | 96.0 | 4.0 |
| Klebsiella pneumoniae (27) | |||||
| Ceftazidime | 32 | >128 | 0.06 to >128 | 37.0 | 63.0 |
| Ceftazidime–avibactam | 0.5 | 4 | ≤0.015–8 | 100 | 0 |
| Cefepime | 64 | >128 | ≤0.06 to >128 | 37.0 | 55.6 |
| Piperacillin/tazobactam | 128 | >128 | 2 to >128 | 40.7 | 55.6 |
| Meropenem | 0.5 | >32 | 0.12 to >32 | 70.4 | 29.6 |
| Colistin | 0.5 | 32 | 0.5 to >32 | 85.2 | 14.8 |
| Amikacin | 2 | 8 | 0.5–32 | 96.3 | 0 |
| Levofloxacin | 16 | >32 | 0.06 to >32 | 40.7 | 59.3 |
| Klebsiella pneumoniae (blaKPC) (30) | |||||
| Ceftazidime | 128 | >128 | 8 to >128 | 0 | 93.3 |
| Ceftazidime–avibactam | 2 | 4 | 0.25–64 | 96.7 | 3.3 |
| Cefepime | 128 | >128 | 2 to >128 | 3.3 | 96.7 |
| Piperacillin/tazobactam | >128 | >128 | 128 to >128 | 0 | 100 |
| Meropenem | >32 | >32 | 8 to >32 | 0 | 100 |
| Colistin | 8 | >32 | 0.5 to >32 | 46.7 | 53.3 |
| Amikacin | 2 | 16 | 1 to >128 | 90.0 | 6.7 |
| Levofloxacin | 32 | >32 | 0.06 to >32 | 6.7 | 93.3 |
| Morganella morganii (25) | |||||
| Ceftazidime | 0.5 | 8 | 0.06–128 | 80.0 | 12.0 |
| Ceftazidime–avibactam | 0.06 | 0.25 | 0.03–2 | 100 | 0 |
| Cefepime | ≤0.06 | 8 | ≤0.06 to >128 | 84.0 | 8.0 |
| Piperacillin/tazobactam | 1 | 8 | 0.25–128 | 92.0 | 4.0 |
| Meropenem | 1 | 1 | 0.5–1 | 100 | 0 |
| Colistin | >32 | >32 | >32 to >32 | 0 | 100 |
| Amikacin | 4 | 8 | 1–8 | 100 | 0 |
| Levofloxacin | 1 | >32 | 0.03 to >32 | 60.0 | 36.0 |
| Proteus mirabilis (25) | |||||
| Ceftazidime | 0.06 | 0.5 | 0.06–8 | 96.0 | 0 |
| Ceftazidime–avibactam | 0.06 | 0.06 | 0.03–0.12 | 100 | 0 |
| Cefepime | 0.12 | 0.5 | ≤0.06–32 | 92.0 | 4.0 |
| Piperacillin/tazobactam | 0.5 | 1 | 0.25–2 | 100 | 0 |
| Meropenem | 1 | 1 | 0.5–2 | 96.0 | 0 |
| Colistin | >32 | >32 | >32 to >32 | 0 | 100 |
| Amikacin | 2 | 8 | 1–128 | 96.0 | 4.0 |
| Levofloxacin | 0.06 | 4 | 0.03–8 | 88.0 | 4.0 |
| Proteus vulgaris (22) | |||||
| Ceftazidime | 0.12 | 0.25 | 0.03–0.5 | 100 | 0 |
| Ceftazidime–avibactam | 0.06 | 0.12 | 0.03–0.25 | 100 | 0 |
| Cefepime | 0.12 | 0.5 | ≤0.06–2 | 100 | 0 |
| Piperacillin/tazobactam | 0.5 | 1 | 0.12–2 | 100 | 0 |
| Meropenem | 1 | 2 | 0.5–2 | 86.4 | 0 |
| Colistin | >32 | >32 | >32 to >32 | 0 | 100 |
| Amikacin | 2 | 4 | 0.5–16 | 100 | 0 |
| Levofloxacin | 0.03 | 0.06 | ≤0.015–4 | 95.5% | 0 |
| Providencia rettgeri (14) | |||||
| Ceftazidime | 0.12 | 0.5 | 0.06–0.5 | 100 | 0 |
| Ceftazidime–avibactam | 0.06 | 0.25 | 0.03–0.5 | 100 | 0 |
| Cefepime | ≤0.06 | 0.12 | ≤0.06–0.12 | 100 | 0 |
| Piperacillin/tazobactam | 1 | 4 | 0.5–4 | 100 | 0 |
| Meropenem | 0.5 | 0.5 | 0.12–0.5 | 100 | 0 |
| Colistin | >32 | >32 | 8 to >32 | 0 | 100 |
| Amikacin | 4 | 8 | 0.5–32 | 92.9 | 0 |
| Levofloxacin | 1 | >32 | 0.12 to >32 | 57.1 | 42.9 |
| Providencia stuartii (22) | |||||
| Ceftazidime | 4 | 16 | 0.25 to >128 | 59.1 | 31.8 |
| Ceftazidime–avibactam | 0.5 | 1 | 0.12 to >128 | 95.5 | 4.5 |
| Cefepime | >128 | >128 | ≤0.06 to >128 | 22.7 | 72.7 |
| Piperacillin/tazobactam | 8 | 64 | 1–128 | 72.7 | 4.6 |
| Meropenem | 0.5 | 0.5 | 0.12–0.5 | 100 | 0 |
| Colistin | >32 | >32 | >32 to >32 | 0 | 100 |
| Amikacin | 2 | 8 | 0.5–64 | 90.9 | 4.6 |
| Levofloxacin | >32 | >32 | 0.25 to >32 | 4.5 | 95.5 |
| Serratia marcescens (26) | |||||
| Ceftazidime | 0.25 | 32 | 0.12 to >128 | 80.8 | 15.4 |
| Ceftazidime–avibactam | 0.25 | 1 | 0.06–32 | 96.2 | 3.8 |
| Cefepime | 0.25 | >128 | ≤0.06 to >128 | 80.8 | 19.2 |
| Piperacillin/tazobactam | 4 | >128 | 2 to >128 | 84.6 | 11.5 |
| Meropenem | 0.5 | 1 | 0.25–4 | 96.2 | 3.8 |
| Colistin | >32 | >32 | >32 to >32 | 0 | 100 |
| Amikacin | 2 | 8 | 1–64 | 92.3 | 3.8 |
| Levofloxacin | 0.25 | 1 | 0.03–4 | 96.2 | 0 |
| Pseudomonas aeruginosa (25) | |||||
| Ceftazidime | 4 | >128 | 0.5 to >128 | 60.0 | 40.0 |
| Ceftazidime–avibactam | 4 | 16 | 0.5–64 | 84.0 | 16.0 |
| Cefepime | 4 | 128 | 1 to >128 | 52.0 | 40.0 |
| Piperacillin/tazobactam | 16 | >128 | 2 to >128 | 60.0 | 28.0 |
| Meropenem | 4 | >32 | 1 to >32 | 32.0 | 32.0 |
| Colistin | 1 | 2 | 0.5–2 | 100 | 0 |
| Amikacin | 4 | 8 | 1–8 | 100 | 0 |
| Levofloxacin | 0.5 | 32 | 0.12–32 | 60.0 | 40.0 |
| Acinetobacter baumannii (50) | |||||
| Ceftazidime | >128 | >128 | 1 to >128 | 18.0 | 82.0 |
| Ceftazidime–avibactam b | >128 | >128 | 2 to >128 | nab | na |
| Cefepime | 64 | >128 | 1 to >128 | 14.0 | 80.0 |
| Piperacillin/tazobactam | >128 | >128 | ≤0.06 to >128 | 14.0 | 82.0 |
| Meropenem | >32 | >32 | 2 to >32 | 16.0 | 84.0 |
| Colistin | 0.5 | 1 | 0.25–32 | 98.0 | 2.0 |
| Amikacin | 64 | >128 | 1 to >128 | 30.0 | 56.0 |
| Levofloxacin | 16 | >32 | 0.06 to >32 | 20.0 | 72.0 |
%S, %R: percent of isolates interpreted as susceptible or resistant. MICs were interpreted according to CLSI criteria (breakpoints),3 except for colistin against Enterobacteriaceae and ceftazidime–avibactam, for which CLSI criteria are not available. MICs of colistin against Enterobacteriaceae were interpreted by EUCAST (European Committee on Antimicrobial Susceptibility Testing) criteria.4 MICs of ceftazidime–avibactam were interpreted according to criteria set by the United States Food and Drug Administration.5 Accordingly, the susceptible and resistant criteria for the Enterobacteriaceae were (mg/L), respectively: ceftazidime, MIC ≤4 and ≥16; ceftazidime–avibactam, MIC ≤8 and ≥16; cefepime, MIC ≤2 and ≥16; piperacillin/tazobactam, MIC ≤ 16 and ≥128; meropenem, MIC ≤ 1 and ≥4; colistin, MIC ≤2 and >2; amikacin, MIC ≤16 and ≥64; and levofloxacin, MIC ≤2 and ≥8. Criteria for susceptible and resistant for P. aeruginosa were (mg/L), respectively: ceftazidime, MIC ≤8 and ≥32; ceftazidime–avibactam, MIC ≤8 and ≥16; cefepime, MIC ≤8 and ≥32; piperacillin/tazobactam, MIC ≤16 and ≥128; meropenem, MIC ≤2 and ≥8; colistin, MIC ≤2 and ≥8; amikacin, MIC ≤16 and ≥64; and levofloxacin, MIC ≤2 and ≥8. The criteria used to interpret MIC values against A. baumannii were identical to those used for P. aeruginosa except for ceftazidime–avibactam, which lacks a breakpoint because the drug label does not include Acinetobacter spp., and colistin, the criterion of resistance to which was MIC ≥4mg/L.
Addition of avibactam at 4mg/L decreased MICs of ceftazidime against unselected Enterobacteriaceae, especially K. pneumoniae, Citrobacter freundii, and Enterobacter cloacae, among which MIC90 values decreased from 128 to >128mg/L to 0.5–4mg/L. Among the unselected isolates of these three species 37–73% were susceptible to ceftazidime, whereas 100% were susceptible to ceftazidime–avibactam. The relatively large decreases in the MIC90 of ceftazidime caused by the addition of avibactam when testing clinical isolates of C. freundii, Enterobacter aerogenes, E. cloacae, Morganella morgannii, Providencia stuartii, and Serratia marcescens likely resulted from the ceftazidime-nonsusceptible isolates of those species producing stably derepressed AmpC β-lactamases.6 This is consistent with the observation that >90% of the isolates of those species remained susceptible to meropenem (Table 1). Of the 27 unselected isolates of K. pneumoniae tested, only 70.4% were susceptible to meropenem, and 37–41% were susceptible to the other β-lactam comparator agents tested, including the β-lactam/β-lactamase-inhibitor combination piperacillin-tazobactam. Susceptibility to levofloxacin was also low, at 40.7%, and almost 15% of the isolates lacked susceptibility to colistin. Of the 30 selected meropenem-nonsusceptible, blaKPC-positive, isolates of K. pneumoniae: none was susceptible to ceftazidime alone, but 29 (96.7%) were susceptible to ceftazidime–avibactam in vitro (MIC90, 4mg/L). As expected, none of the β-lactam agents (except ceftazidime–avibactam) displayed appreciable activity against these isolates, and even susceptibility to colistin was compromised at 46.7% (Table 1). Susceptibility to amikacin was 90.0% (Table 1). This level of susceptibility of the blaKPC-positive isolates to ceftazidime–avibactam is consistent with the results of the global surveillance studies of carbapenem-resistant K. pneumoniae.2 In the case of E. coli, the range of MICs showed the avibactam effect, 0.12–64mg/L for ceftazidime as opposed to 0.06–0.25mg/L for ceftazidime–avibactam. Little to no avibactam effect was observed when testing ceftazidime–avibactam as opposed to ceftazidime against Citrobacter koseri, Klebsiella oxytoca, Proteus vulgaris, or Providencia rettgeri, as all isolates were already susceptible to ceftazidime.
Addition of avibactam decreased the MIC90 of ceftazidime against 25 unselected isolates of P. aeruginosa from >128mg/L to 16mg/L, resulting in 84% of isolates being interpreted as susceptible in vitro (Table 1), indicating a slightly lower level of susceptibility than has been found among isolates of this species in global surveillance studies. For example, in one recent global study, the MIC90 of ceftazidime–avibactam against 7062 isolates of P. aeruginosa was 8mg/L, and 92% were susceptible.7 Avibactam inhibits the AmpC β-lactamase of P. aeruginosa and restores susceptibility to ceftazidime in isolates in which the mechanism of resistance is that of stable derepression of that enzyme.8 However, multidrug-resistant P. aeruginosa clone ST277 that frequently harbors metallo-β-lactamase SPM-1 is disseminated widely in Brazil8 and avibactam does not inhibit metallo-β-lactamases, which might explain the 16% resistance to ceftazidime–avibactam found here (Table 1). Nevertheless, the percent of the P. aeruginosa isolates in the present study that were susceptible to ceftazidime–avibactam was higher than the percent that were susceptible to the other β-lactam agents tested, including meropenem (32% susceptible) and piperacillin/tazobactam (60% susceptible) (Table 1).
The MIC90 of ceftazidime against 50 isolates of Acinetobacter baumannii was >128mg/L whether avibactam was present or not, which is consistent with this species not being listed on the drug label.5
In conclusion, the in vitro antibacterial activity of ceftazidime–avibactam against bacteria isolated from patients in Brazil was consistent with results from other surveillance studies except that the percent susceptibility of the sample of clinical isolates of P. aeruginosa at 84% (Table 1) was somewhat lower than the proportions observed elsewhere,7 possibly related to the dissemination of the metallo-β-lactamase, SPM-1, in Brazil.8 The in vitro activity of ceftazidime–avibactam described in the present work is consistent with its recently-reported efficacy in phase 3 clinical studies.9
Conflicts of interestWW Nichols and R Testa were employees of AstraZeneca at the time of the study and WWN is an AstraZeneca shareholder. Pfizer acquired the AstraZeneca product, ceftazidime-avibactam, in December 2016. The Hospital das Clinicas da Universidade de São Paulo Group declare no conflicts of interest.
We thank AstraZeneca for funding this study and TR Di Gioia and J Nobrega Jr for technical assistance.