The COVID-19 pandemic has triggered crises in the public health sector that have complex and multifaceted interrelationships with antimicrobial resistance. It is important to evaluate the impact of COVID-19 on microbiological profile, antibiotic and alcohol gel consumption in Intensive Care Units (ICU).
MethodsThis is a retrospective study undertaken in an infectious disease hospital located in Bahia/Brazil during three periods: from March 2019 to February 2020; from March 2020 to February 2021; and from March 2021 to February 2022. It was evaluated the incidence density of Candida spp and of multidrug-resistant Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species (ESKAPE group) in blood, urine and tracheal secretion isolated 48 h after the patient's admission to the ICU, as well as the use of alcohol gel (in milliliters) and consumption of antibiotics in Defined Daily Dose (DDD) per 1,000 ICU patient-days in the previous year and in the first two years of COVID-19 pandemic.
ResultsThere was an increase in Candida spp. (5.81, p < 0.001, IRR = 10.47, 95 % CI 2.57‒42.62) and in carbapenem-resistant A. baumannii in clinical cultures (4.71, p < 0.001, IRR = 8.46, 95 % CI 2.07‒34.60), the latter mainly in tracheal secretions (3.18, p= 0.02, IRR = 11.47, 95 % CI 1.58‒83.39). A rise in the consumption of ceftriaxone and piperacillin-tazobactam, along with an increase in the utilization of alcohol gel were observed.
ConclusionThe shifting microbiological profile can be attributed to both the unique characteristics of patients with COVID-19 and the adjustments made to healthcare facilities' structural and work routines. Understanding these changes is essential in addressing the accelerated impact of antimicrobial resistance during the pandemic. Therefore, conducting thorough reviews of institutional practices and routines becomes critical in mitigating the consequences of antimicrobial resistance and its implications for patient care.
According to a report published before the COVID-19 pandemic, by the year 2050, it is expected that we will reach 10 million deaths per year as a result of antimicrobial pathogens infections, which surpasses the amount of deaths from cancer, diabetes, and accidents.1 The prolonged Intensive Care Unit (ICU) hospitalization and the extensive use of broad-spectrum antimicrobial drugs in COVID-19 patients might have contributed to the selection of pathogens with different profiles of resistance, which may perhaps bring the consequences expected for 2050 closer. Around 70 % of COVID-19 patients admitted to hospitals received antibiotic treatment, mostly azithromycin and ceftriaxone.2
The pandemic has triggered crises in the public health sector that have complex and multifaceted interrelationships with antimicrobial resistance.3 Some of the effects of intense COVID-19 caseloads on hospitals could potentially impact infection prevention control practices, such as burnout risk among healthcare workers associated with a patient-to-nurse ratio over 2:1, higher workloads, deaths of COVID-19 patients and a shortage of personal protective equipment.4 The overcrowding of hospitals has led to a breakdown in infection control measures and antimicrobial stewardship activities in some institutions that may have led to multidrug-resistant bacteria outbreaks, which increases the need for broad-spectrum antimicrobials prescription.5
In hospitalized critically ill COVID-19 patients, the incidence of secondary infections with Multidrug-Resistant (MDR) bacteria ranged from 30 %‒50 %, with the majority being respiratory tract and bloodstream infections, 10‒15 days after admission, mainly by carbapenem-resistant Enterobacterales and P. aeruginosa.6 It is possible that the high intensity of care, the need to be changed in a prone position by 4–5 healthcare workers equipped with personal protection equipment in a high-risk area with extended and prolonged contact with the patient, and the presence of health personnel without work experience in ICU settings regarding contact precautions, contributed to the increased in carbapenem-resistant Enterobacterales acquisition colonization in COVID-19 patients.7
According to data prior to the COVID-19 pandemic, globally, the leading cause of hospital-acquired infections are the so-called ESKAPE pathogens: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species.8 It is therefore important to verify the impact of the pandemic on the antimicrobial susceptibility profile of these microorganisms in health services in order to identify priorities for action by infection control services.
This is a retrospective study that evaluated the incidence density of Candida spp. and MDR bacteria from the ESKAPE group, as well as the use of alcohol gel and antibiotics in the previous year (2019) and in the first two years (2020‒2021) of the pandemic in ICUs of a specialized hospital. The primary objective of the study is to compare the microbiological profile of ICUs before and during the first two years of the COVID-19 pandemic. Secondarily, the use of alcohol gel used in hand hygiene and the consumption of antibiotics prescribed in the ICU were also evaluated.
MethodsInstituto Couto Maia (ICOM) is a hospital specializing in infectious diseases situated in Bahia, Brazil. On March 17th, 2020, ICOM underwent a significant transformation and became an exclusive reference hospital dedicated to providing care for patients diagnosed with COVID-19. To meet the escalating demands of the pandemic, the hospital swiftly expanded its capacity from 126 to 162 beds, including the addition of 70 ICU beds. Prior to the onset of the pandemic, ICOM operated with a 20-bed ICU. However, in response to the pandemic's unprecedented challenges, the hospital underwent necessary adjustments. It reconfigured existing wards, transforming them into five separate intensive care units, each exclusively designed to accommodate adult patients, with each unit housing 10-beds.
At ICOM, a retrospective analysis was undertaken comprised three different periods: the first from March 2019 to February 2020 (pre-pandemic period); the second from March 2020 to February 2021; and the third from March 2021 to February 2022. The objective was to determine the incidence density of MDR bacteria and Candida spp. in blood, urine, and tracheal secretion cultures per 1000 ICU patient-days.
Additionally, the consumption of antimicrobial was calculated based on the Defined Daily Dose (DDD), and the consumption of alcohol gel was calculated in milliliters, both per 1000 patient-days in the ICU. The pre-pandemic period (March 2019 to February 2020) served as a reference to compare and evaluate the incidence density of microorganisms over the subsequent years.
Antimicrobial-resistant ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) were assessed if detected within 48 h after the patient's admission to the ICU. In addition to ESKAPE pathogens, the evaluation also included E. faecalis and Candida spp.
The incidence density of carbapenem-resistant K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter spp.; as well as Vancomycin-Resistant E. faecalis and E. faecium (VRE spp.) and oxacillin-resistant Staphylococcus Aureus (MRSA) were analyzed. Concerning Candida spp., the incidence density was evaluated regardless of the susceptibility profile. Due to the limited number of cases, all vancomycin-resistant Enterococcus species were grouped together for analysis. Cultures from the same patient with the same pathogen and antimicrobial susceptibility profile were excluded, considering only one for evaluation. Pediatric units were not evaluated.
The microbiological analysis was performed using automated microdilution methods using the Vitek®2 BioMérieux system, as well as disk diffusion using the BrCAST cut-off points. Data analysis was conducted using the Epi-info v.3.5.1 program (CDC/USA). The results were considered statistically significant when the p-value was less than 0.05, with a 95 % Confidence Interval (95 % CI).
Open-Source Epidemiologic Statistics for Public Health software (version 3.01, available at https://www.openepi.com/Menu/OE_Menu.htm) was employed for the statistical analysis. For the purpose of comparison, the pre-pandemic period was defined as the 1-year period between March 2019 and February 2020. During the pandemic, two distinct periods were considered: March 2020 to February 2021 (first period) and March 2021 to February 2022 (second period).
To calculate the incidence per 1000 ICU-patient-days, the number of MDR bacteria and Candida spp isolates were used as the numerator, while all inpatients served as the denominator. The incidence was then estimated for each year from 2019 to 2022 based on the predefined periods. Furthermore, the Incidence Rate Ratios (IRRs) with 95 % Confidence Intervals (95 % CI) were employed to compare the incidence rates during each pandemic period to those observed in the pre-pandemic period utilizing Fisher's exact test for statistical inference.
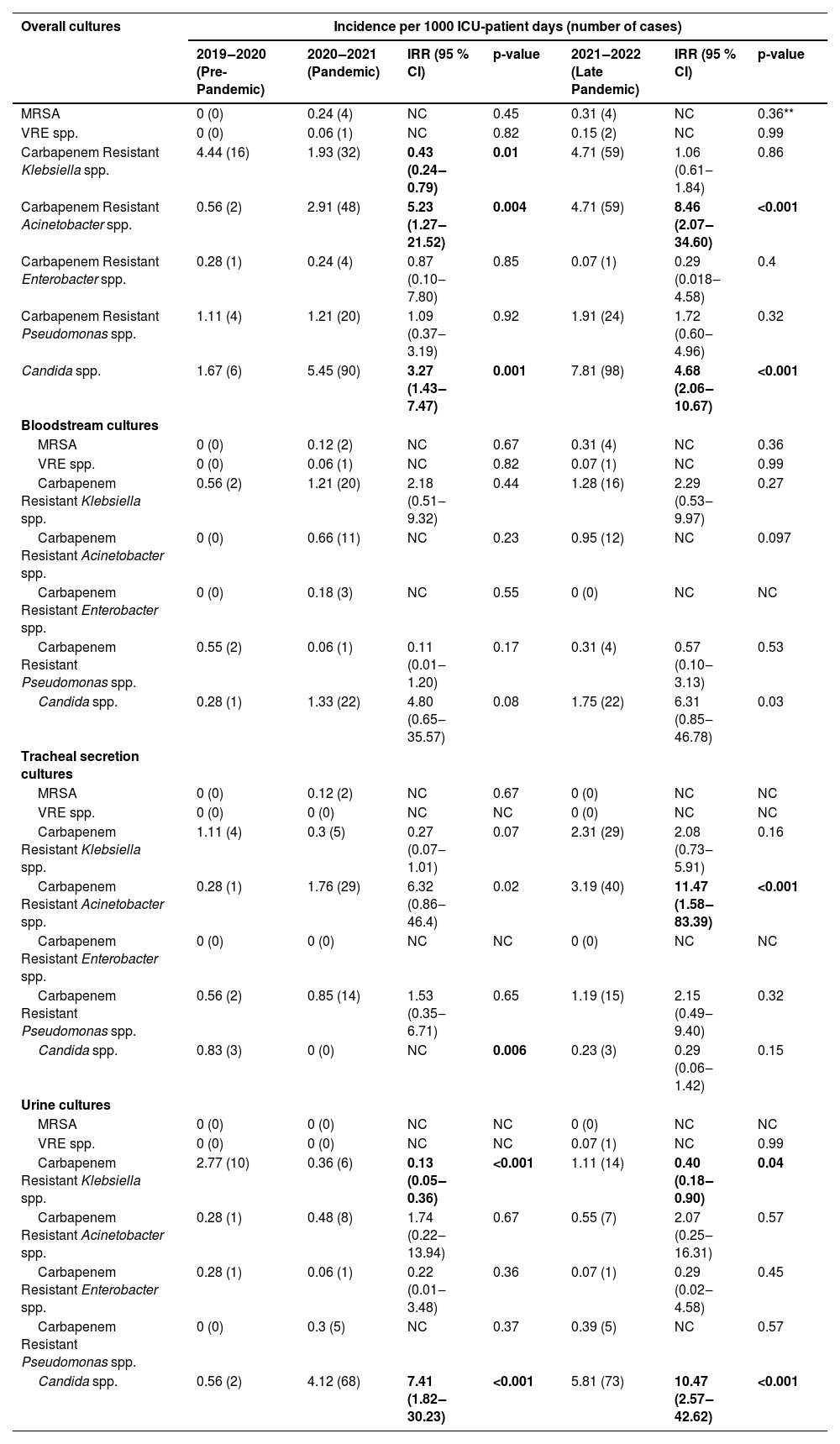
ResultsThe total ICU patient days for the respective periods were as follows: from March 2019 to February 2020 (pre-pandemic period) ‒ 3598 ICU patient days; from March 2020 to February 2021 (first period) ‒ 16,502 ICU patient days; and from March 2021 to February 2022 (second period) ‒ 12,548 ICU patient days. The incidence density of MDR bacteria in total cultures (blood cultures, tracheal secretions and urine cultures) during the three periods were evaluated using the pre-pandemic period as a reference for comparison to assess the incidence density in the subsequent years and the results were: oxacillin-resistant S. aureus 0; 0.24 (p = 0.45) and 0, 31.0 (p = 0.36), vancomycin-resistant Enterococcus spp. 0; 0.06 (p = 0.82) and 0.15 (p = 0.99), carbapenem-resistant K. pneumoniae 4.44; 1.93 (p = 0.0)1 and 4.71 (p = 0.86), carbapenem-resistant A. baumannii 0.56; 2.91 (p = 0.004) and 4.71 (p < 0.001), Candida spp. 1.67; 5.45 (p = 0.001) and 7.81 (p < 0.001) (Table 1).
Incidence density rate per microorganisms, stratified by study period and different body site.
Table footnote: The bolding formatting is signaling statistical significance.
The numbers in the lines represent the incidence density of MDR and of Candida spp. in each period.
During the pandemic, it was observed that the increase in the incidence density of carbapenem-resistant A. baumannii was due to its progressive rise in the tracheal secretion. Specifically, in this site, the incidence density from March 2019 to February 2020 (pre-pandemic period) was of 0.28, which increased to 1.76 in the second period (p = 0.02, IRR = 6.32, 95 % CI 0.86‒46.4), and further rose to 3.18 in the third period (p = 0.02, IRR = 11.47, 95 % CI 1.58‒83.39). The incidence density per culture site of all microorganisms evaluated in the study is provided in Table 1.
The increase in incidence density of Candida spp. was primarily attributed to urinary colonization, which increased from 0.56 in the pre-pandemic period to 4.12 in the first period (p < 0.001, IRR = 7.41, 95 % CI 1.82‒30.23) and further elevated to 5.81 in the second period (p < 0.001, IRR=10.47, 95 % CI 2.57‒42.62). Regarding candidemia, the incidence density also showed an increase from 0.28 in the first period to 1.33 in the second period. However, this change did not reach statistical significance (p = 0.08, IRR = 4.80, 95 % CI 0.65‒35.57). An even more substantial increment in the incidence density was observed in the second period (March 2021 to February 2022) compared to the pre-pandemic period (March 2019 to February 2020), with an increase from 0.28 to 1.75 (p = 0.03, IRR = 6.31, 95 % CI 0.85‒46.78); although, it also did not reach statistical significance. It is important to note that Candida auris was not isolated during any of the three periods under investigation.
The consumption of the antibiotics, measured in grams per 1000 ICU patient-days, during the three evaluated periods were as follows: ceftriaxone 170.9; 670.82, and 377.43, respectively; ciprofloxacin 50.86; 11.27 and 37.75, respectively; meropenem 1174.54; 848.32 and 1198.88, respectively; piperacillin-tazobactam 90.6; 389.4 and 159.56, respectively; vancomycin 219.01; 373.16 and 319.17 respectively. Regarding the use of alcohol gel, measured in milliliters per 1000 ICU patient-days, in chronological order, the totals were: 20.08, 33.35, and 44.27.
DiscussionIn this study, during the first two years of the pandemic, there was an increase in Candida spp. and in carbapenem-resistant A. baumannii, with the latter primarily found in tracheal secretions. Carbapenem-resistant A. baumannii is an opportunistic pathogen commonly associated with healthcare-associated infections in COVID-19 patients; particularly affecting the lower respiratory tract and exacerbating the patients' condition.9,10 Studies from other institutions worldwide have reported the incidence of secondary infections due to A. baumannii to be around 1 % in hospitalized COVID-19 patients.11-13 Interestingly, during the first two years of the COVID-19 pandemic, an outbreak of carbapenem-resistant A. baumannii ventilator-associated pneumonia was detected in the ICUs of our hospital. This outbreak may account for the observed increase in carbapenem-resistant A. baumannii in tracheal secretions in the current study.
The rise in carbapenem-resistant A. baumannii during the COVID-19 pandemic, compared to pre-pandemic period (March 2019 to February 2020), may not be attributed to antibiotic overuse in our study, as the consumption of meropenem remained stable. Interestingly, during the same period, the use of alcohol gel increased from 20.08 to 44.27 mL/patient, indicating a potential improvement in hand hygiene practices. However, this information only pertains to the use of alcohol gel for hand hygiene and does not address whether it was appropriately applied in all hand hygiene opportunities or potentially misused, such as for non-recommended and ineffective practices like glove hygiene.
The elevated detection of carbapenem-resistant A. baumannii in tracheal secretions might be indicative of errors in routines and processes associated to mechanical ventilation and airway management in critically ill patients. Further investigations are necessary to better understand the specific factors contributing to this increase and identify potential areas for improvement in infection control practices during the pandemic.
The COVID-19 pandemic has posed unprecedented challenges for hospitals, necessitating innovative measures to ensure continuous patient care, especially in the face of limited access to supplies and healthcare professionals on a global scale. In response, our hospital, along with other institutions facing carbapenem-resistant A. baumannii outbreaks14 implemented various adaptations in infection prevention and control practices during the pandemic. In order to prioritize personnel resources, hospitals temporarily discontinued activities of the multidrug-resistant microorganisms’ workgroups responsible for guiding infection prevention and control practices. These workgroups, which typically conduct routine audits on the appropriate use of Personal Protective Equipment (PPE), hand hygiene compliance, and environmental cleaning, had their activities interrupted. Additionally, surveillance cultures were reduced during this period.
Efforts were made to conserve PPE throughout the working hours and implement patient care strategies aimed at minimizing healthcare personnel exposure. Furthermore, patient surge staffing protocols were put in place to manage the influx of COVID-19 cases. These adaptations might have inadvertently contributed to an increased likelihood of a carbapenem-resistant A. baumannii outbreak in a COVID-19 intensive care unit in the United States.15
In our study, although there was an increase in the identification of Candida spp. in clinical cultures during the first two years of the pandemic, the elevation of the risk of candidemia showed variable results, ranging from 4.8 to 6.31 times, but did not reach statistical significance. Similar observations, of increased candidemia was described by Nucci et al. (2021) in another hospital in Brazil, coinciding with the admission of patients with COVID‐19.16 Additionally, a study carried out in a tertiary hospital in Spain identified two significant increases in candidemia, including cases caused by C. auris, during the first and third waves of the pandemic.17
The potential rise in candidemia could be related to the characteristics of the COVID-19 patients, such as immune dysfunction, lymphopenia, and underlying comorbidities, as well as the treatment they receive, such as, corticosteroids, Interleukin 6 (IL-6) inhibitors, dialysis, mechanical ventilation, invasive devices, and the use of broad-spectrum antibiotics.18,19 Regarding Candida spp. detected in urine, in our study, were considered colonization, possibly associated with the increased use of ceftriaxone and piperacillin-tazobactam.
Before the COVID-19 pandemic, our hospital had no records of infection or colonization in clinical cultures with Vancomycin-Resistant Enterococcus spp. (VRE). However, during the pandemic, VRE infections were identified as the cause of some catheter-related bloodstream and urinary tract infections. A similar nosocomial cluster of VRE was reported in a German hospital during the pandemic, where reduced personal staffing was associated with poor adherence to infection control measures. Whole genome sequence-based typing, combined with epidemiological data analysis, indicated that contaminated surfaces also played a role in ongoing transmission during this cluster. This published study emphasizes the critical importance of maintaining stringent adherence to infection prevention and control measures during the COVID-19 pandemic to effectively prevent VRE transmission and healthcare-associated infections.20
In our study, a notable rise in the consumption of ceftriaxone and piperacillin-tazobactam was observed, particularly among patients admitted to the ICU from the community or emergency health units. However, a review of bacterial and fungal coinfection in individuals with coronavirus at hospital admission showed that only 8 % of patients were reported as experiencing coinfection during hospital admission. Despite the frequent prescriptions of broad-spectrum empirical antimicrobials in patients with coronavirus-associated respiratory infections, there is a lack of sufficient data to definitively establish the association with respiratory bacterial/fungal coinfections.21,22
This study is conducted at a single center and, in some periods, the number of cases is limited, which may have impacted the accuracy of the findings. Additionally, the COVID-19 pandemic could have introduced changes in diagnostic routines, potentially affecting the identification of certain bacteria. Another limitation of our study is that it focused on determining the microbiological profile rather than solely isolates related to hospital-acquired infections. Consequently, the analysis includes isolates related to colonization, not exclusively infection cases. This aspect should be considered when interpreting the results and drawing conclusions from the study
ConclusionAmidst the pandemic, the ICU of a specialized COVID-19 hospital witnessed a rise in the detection of carbapenem-resistant A. baumannii, despite the increased use of alcohol gel and no significant change in meropenem consumption. Additionally, there was an increase in Candida spp. identified in clinical cultures during the initial two years of the pandemic. These alterations in the ICU's microbiological profile can potentially be attributed to the unique patient profile associated with COVID-19 and the structural and work routine adaptations made during the pandemic. Understanding these factors is crucial in comprehending the changes in antimicrobial resistance within healthcare settings.
A thorough evaluation of practices and routines adopted by institutions can play a pivotal role in mitigating the impact of accelerated antimicrobial resistance during the pandemic. By proactively reviewing and adjusting protocols, healthcare facilities can effectively address the challenges posed by increased antimicrobial resistance, safeguarding patient well-being, and optimizing treatment outcomes.