To study the human migration inhibitory factor (MIF) level in tuberculosis (TB) patients, and the relationship between MIF-794CATT microsatellite polymorphism and susceptibility of TB in Southwest China.
MethodsTB patients (n=151) and healthy unrelated controls (n=149) were recruited for this study. Genomic DNA was extracted, and then amplified by polymerase chain reaction (PCR). MIF-794CATT5-8 microsatellite polymorphism was genotyped by DNA sequencing. MIF level was detected by ELISA.
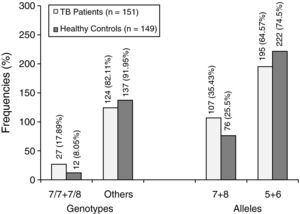
ResultsIn the TB group, the repeat number of 7/7 and 7/8 (17.89%) was significantly higher than that of the control group (8.05%), and the serum MIF level was also much higher than that of the healthy controls (705.21±67.98 vs. 355.31±57.29 pg/mL, p<0.01).
ConclusionThe appearance of MIF-794CATT 7/7 and 7/8 is associated with susceptibility to TB, and may play an important role in the occurrence and development of TB in Southwest China.
Tuberculosis (TB), which is a chronic infectious disease caused by Mycobacterium tuberculosis (MTB), is a serious threat to human health. According to the WHO survey, there are an estimated 14 million TB patients globally, while nearly 1.7 million people die of TB each year.1 Currently, about 130,000 people die of TB and 1.45 million new patients are diagnosed with TB each year in China, which has the second highest number of TB patients in the world. Thus, prevention and control of TB is attracting more and more attention of the medical world.
Studies have confirmed that besides environmental factors and individual differences, genetic susceptibility genes also play a role in the development of TB.2 The study by Fontaine et al. found that human migration inhibitory factor (MIF) genetic variants were associated with susceptibility to autoimmune disease.3 In an in vitro study, Gómez et al. reported that MIF played an important role in inhibiting the growth of pathogenic MTB in macrophages by acting in an autocrine fashion.4 The MIF gene is located on human chromosome 22q11.2. Two polymorphisms with potential functional relevance have been reported in the MIF promoter: SNP -173G/C and microsatellite polymorphism -794CATT5-8. The C genotype of MIF-173 was found in this study to be associated with TB. The relationship between MIF-794CATT microsatellite polymorphism and susceptibility of TB was further investigated in the present study.
Materials and methodsSubjectsCases were recruited from patients who were diagnosed with TB at the Third Affiliated Hospital of the Third Military Medical University and at the TB Prevention and Treatment Center in Chongqing (Chongqing, China) from December, 2009 to December, 2010. 151 TB patients (83 males and 68 females, mean age 47.8±5.76 years) and 149 healthy unrelated controls (61 males and 88 females, mean age 40.2±4.9 years) were included in the present study, and everyone in the control group was in-patient at the Third Affiliated Hospital of the Third Military Medical University. Diagnosis of TB was based on the identification of acid-fast bacilli (AFB) in sputum samples and chest X-ray examination. Exclusion criteria were: hypertension, diabetes, thyroid disease, liver disease, kidney disease, cancer, chronic wasting disease, and infection of the heart, brain, kidney, or lung.
Human genomic DNA extractionGenomic DNA was extracted from peripheral blood anticoagulant with EDTA-K2 by a commercially available blood DNA extraction kit (Tiangen Company, Beijing, China) according to instructions. Then the isolated DNA was stored in TE solution, frozen at -20°C for use.
MIF-794CATT5-8 genotypingPrimers were designed to amplify a 169bp (CATT6) segment of the MIF promoter region containing the microsatellite repeat sequence. The upstream primer was 5’-CTATCAGAGACCAAGGACAG-3’, and the downstream primer was 5’ -CCAGGCATATCAAGAGACAT-3’. The PCR reaction conditions were as follows: denaturation at 95°C for 10min, 35 cycles of 95°C for 45sec, 57°C for 40sec, 72°C for 45sec, followed by a seven-minute extension at 72°C. The unpurified PCR products were sent directly to the Biology CRO of the Nanjing GenScript Corporation for sequencing. The sequencing primers were the same as those of PCR.
Analysis of serum MIF levelsBlood serum was separated from 3mL of peripheral blood from the TB patients and from the control group, and then stored at -20°C for MIF levels testing with ELISA kits (R&D, USA).
Statistical analysisThe sequencing results were analyzed by Primer 6.0. Hardy-Weinberg equilibrium was used for testing the allele and genotype distribution. The frequencies for the TB patients and control-group individuals were statistically compared using the chi-squared test with the Statistical Package for Social Sciences (SPSS) 17.0 software, and MIF levels were compared using Student's t-test. A p-value<0.05 was considered statistically significant.
ResultsMIF-794CATT5-8 polymorphism analysisPeripheral blood genomic DNA was extracted from 151 confirmed TB patients and 149 controls. The sequencing analysis results of the MIF gene promoter -794 polymorphism are shown in Fig. 1. The results reveal that there are five to eight CATT repeats sequenced in each single-stranded DNA. The frequency distribution of the MIF gene -794CATT5-8 allele is shown in Fig. 2, which shows that the percentage of the MIF -794CATT with the repeat number 7/7 and 7/8 is 17.89% in TB group patients, which is significantly higher than the 8.05% of the control group (p<0.05, OR=2.49).
Serum MIF levelsThe serum MIF levels of TB patients and normal controls were 705.21±67.98 and 355.31±57.29 pg/mL, respectively. The average MIF level of the TB patients was almost twice as high as that of the healthy control group (p<0.01).
DiscussionGene polymorphism plays an important role in the occurrence and development of TB. Several TB susceptibility genes have been discovered, such as natural resistance associated macrophage protein-1 (Nramp1) gene,5 human leukocyte antigen (HLA) gene,6 vitamin D receptor (VDR) gene,7 SP110 nuclear body protein gene (SP110),8 mannose binding lectin (MBL) gene,9 nitric oxide synthase 2 gene (NOS2A),10 and IFN-γ gene (IFNG).10 Therefore, studies on the relationship between gene polymorphism and TB susceptibility are of great significance in the prevention and treatment of TB.
MIF is a protein consisting of 115 amino acids with a molecular weight of 12.5 kDa. Because of its wide expression in various cells, MIF is considered to be a versatile cytokine. Recently, many studies have reported that MIF is not only a T cell-derived cytokine, but could also be released from other cells such as monocyte-macrophage cells and anterior pituitary cells. The results of this study confirm that MIF-794CATT 7/7 and 7/8 genotypes are associated with the susceptibility of TB (OR=2.49). The ratio of the genotype CATT5+6 in TB patients group is 64.57%, which is lower than the control group (74.5%), indicating that CATT5+6 may inhibit the susceptibility of TB.
This study also confirms that the MIF level of TB patients is significantly higher than that of the healthy controls. The MIF-794CATT5-8 microsatellite polymorphism has been found to be associated with the alteration of MIF gene transcription levels in vitro.11 The repeat number of the CATT can regulate the activity of MIF gene promoter. The higher the repeat number, the stronger the activity of the promoter. In vitro, MIF can inhibit the growth of pathogenic Mycobacterium tuberculosis.12 However, it is speculated that, when the body is infected with M. tuberculosis, it is supposed to be any mechanisms which could induce MIF loss or weaken the inhibition of M. tuberculosis. In short, the mechanism of MIF in the development of TB remains unclear, and further experimental studies are still needed to confirm it.
Conflict of interestAll authors declare to have no conflict of interest.
This work was supported by the National Infection Disease Prevention and Cure Special Project of China (2008ZX10003012).