Latin America is the region with the third most AIDS-related cryptococcal meningitis infections globally. Highly active antiretroviral therapy (HAART) has reduced the number of infections; however, the number of deaths and the case-fatality rate continues to be unacceptable. In this review, we focus on the burden of AIDS-related cryptococcosis in Latin America and discuss potential strategies to reduce early mortality from Cryptococcus. In this review, we highlight the importance of: (1) earlier HIV diagnosis and HAART initiation with retention-in-care to avoid AIDS; (2) pre-HAART cryptococcal antigen (CRAG) screening with preemptive fluconazole treatment; (3) better diagnostics (e.g. CRAG testing); and (4) optimal treatment with aggressive management of intracranial pressure and induction therapy with antifungal combination. Implementation of these strategies can reduce cryptococcal-related deaths, improve care, and reduce healthcare costs.
Cryptococcal meningitis affects approximately 1,000,000 people in the world each year and results in more than 400,000 deaths within three months after disease.1 Sub-Saharan Africa had the highest burden with an estimated yearly cryptococcal meningitis cases: 720,000, but Latin America is the third global region with most cases with 54,400 estimated cryptococcal meningitis cases annually. Most published studies regarding cryptococcal meningitis have been reported from sub-Saharan Africa, South Asia, and Southeast Asia,1–6 and data are scarce from Latin America.
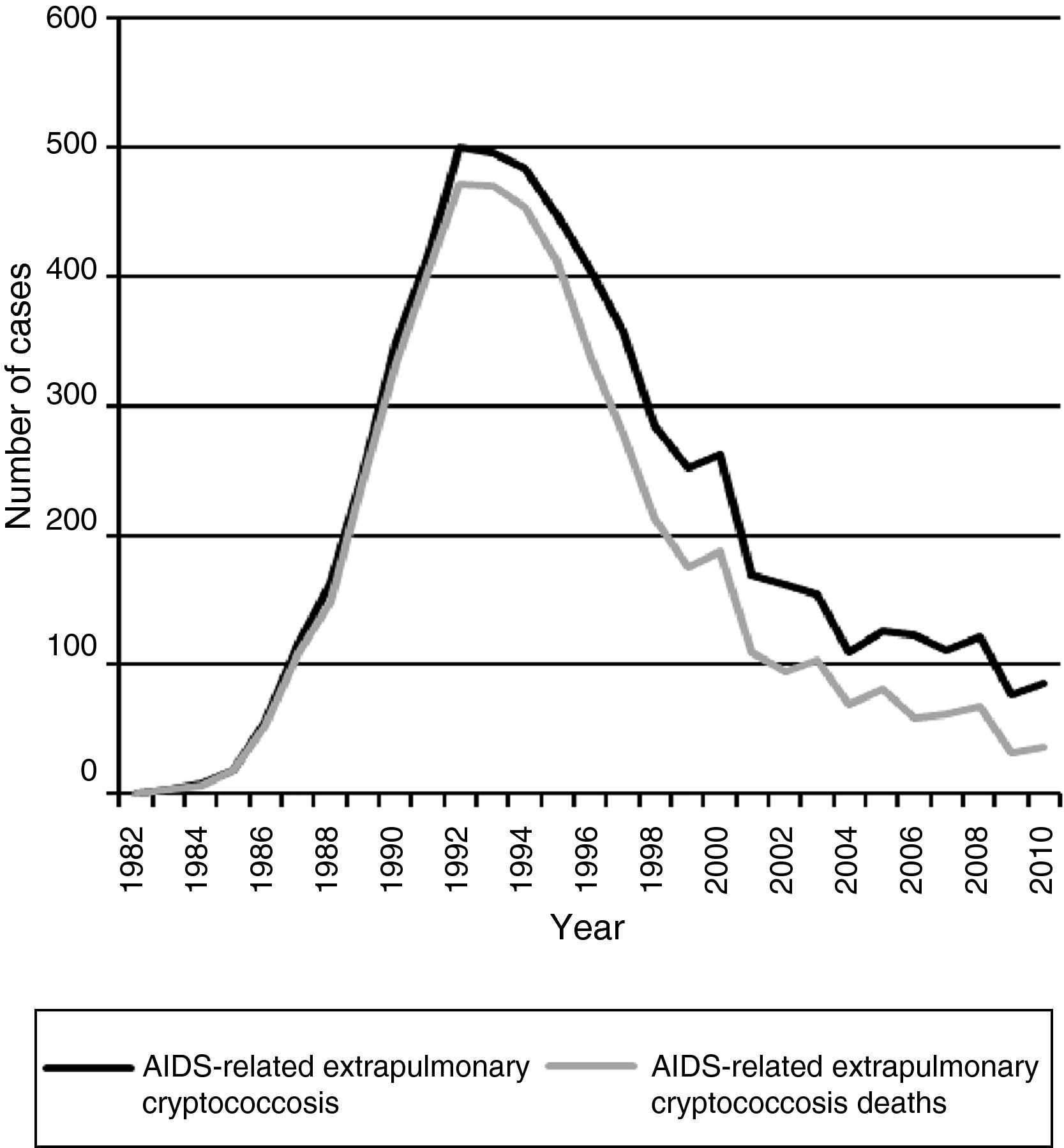
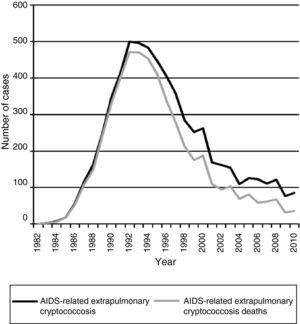
Acquired immunodeficiency syndrome (AIDS)-associated cryptococcal meningitis has decreased dramatically in high-income countries in the highly active antiretroviral therapy (HAART) era.7–9 However, the impact of HAART in reducing cryptococcosis appears to be less in low and middle-income countries with suboptimal access to HAART.10–12 Some studies suggest a declining number of cases in Brazil.10,13 Data obtained from the Epidemiology Service of the Centro de Referência em Treinamento em DST/AIDS from São Paulo, showed a progressive and important declining of notified cases of AIDS-related extrapulmonary cryptococcosis during the HAART era. Fig. 1 displays the decline in reported cases from 1982 to 2010 with 500 reported cases in 1992 and declining to only 82 cases in 2010 reported from São Paulo. This is in the setting of a well-structured HAART access. Certainly, the magnitude of this result cannot be generalized to all cities or rural regions in Brazil.14
Currently, cryptococcal meningitis is not an uncommon complication in Brazil and other Latin American countries. In this region, cryptococcosis represents the main cause of opportunistic meningitis,15–18 and the mortality continues to be unacceptably high (∼55%).1 Cities in Latin America are very heterogeneous, and populations have substantial socioeconomic disparities. In this scenario, the human immunodeficiency virus (HIV) epidemic and opportunistic infections disproportionally affect persons with lower socioeconomic status.
In this review, we discuss the mortality of AIDS-related cryptococcal meningitis in Latin America, mortality in high versus low and middle-income settings, prognostic factors associated with mortality, and strategies to reduce cryptococcal-related mortality in the region.
Mortality of AIDS-related cryptococcal meningitis in Latin AmericaThere is limited information about case-fatality rate of HIV-infected patients with cryptococcal meningitis in Latin America in routine practice where amphotericin B is available but not 5-flucytosine (5-FC) and where combination induction therapy with amphotericin and fluconazole is yet infrequent.
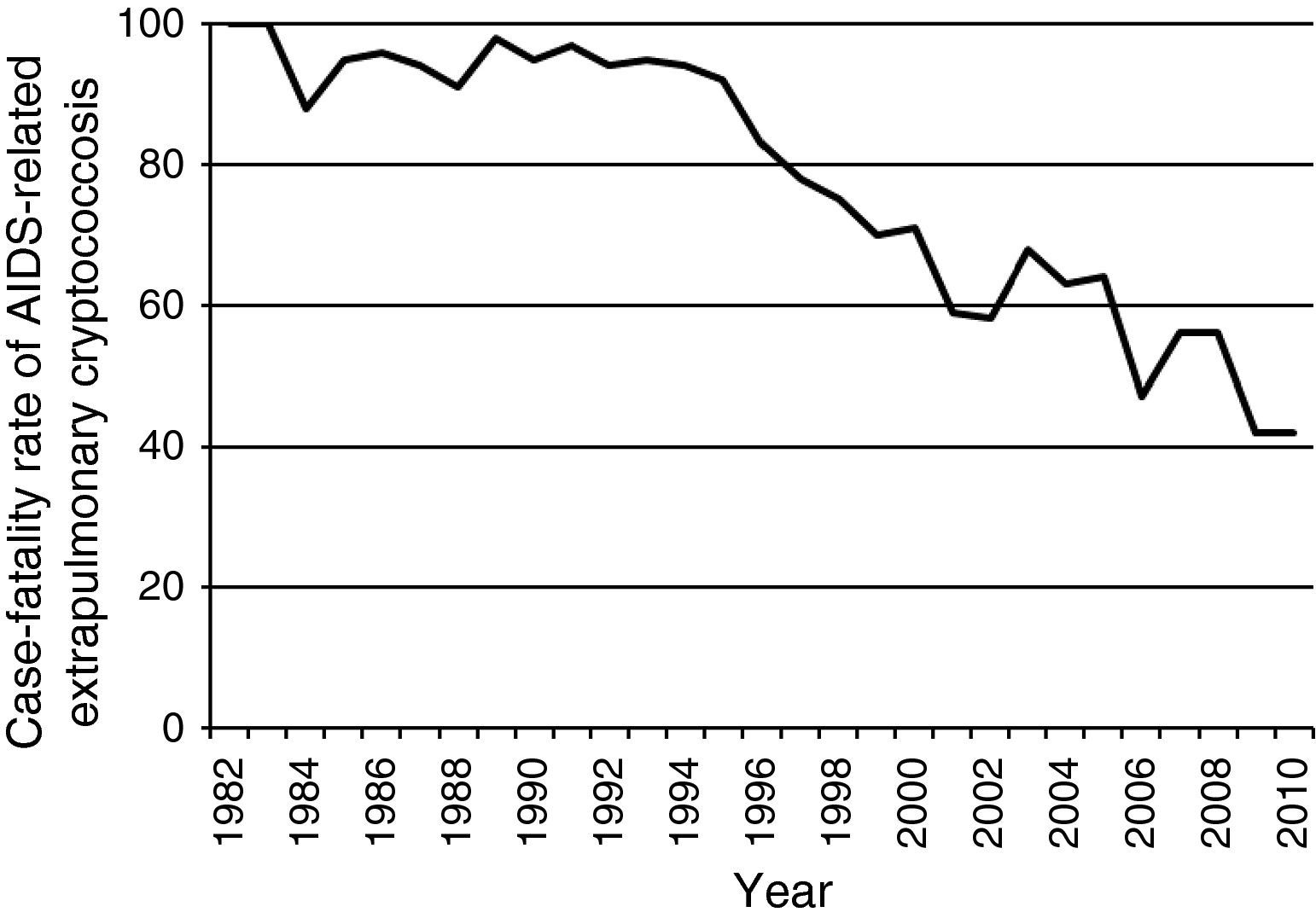
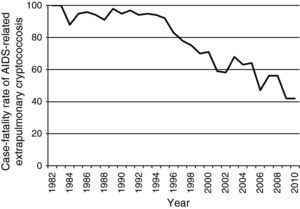
Data from the Epidemiology Service of the Centro de Referência em Treinamento em DST/AIDS from São Paulo show a consistent decline in notified cases of extrapulmonary cryptococcosis-related deaths among AIDS patients during the HAART era (Fig. 1). In addition, there has been a reduction in the case-fatality rate of extrapulmonary cryptococcosis from >90% in the pre-HAART era to ∼40% in the HAART-era (Fig. 2). Nevertheless, the burden of cryptococcal deaths continues.
In unselected retrospective hospital-based studies performed in Brazil and Argentina, the case fatality rates have ranged from 30% to 63%.19–24 In most of these studies increased intracranial pressure (ICP) was not systematically treated. A recent prospective hospital-based study performed in Brazil reported a case fatality rate of 50% among 131 patients between 1998 and 2010.25 Conversely, two prospective studies reported lower mortality. The first, a Peruvian study treating patients with amphotericin B plus aggressive management of ICP in the setting of a research study, the 10-week mortality was 19%.26 The second, a Brazilian non-published prospective study with 34 patients performed in the HAART era in which all cases received amphotericin B plus aggressive management of ICP, the 10-week mortality was 26%.27 Taken together, these studies confirm that acute mortality of AIDS-related cryptococcal meningitis is high in Latin America. The mortality is similar to 24–50% reported in interventional studies carried out in Africa and Asia.28–31 In high resource countries, mortality ranges from 9% to 25%.32–37 Interestingly, a recent Italian study reported a 10-week mortality of only 1 (2.5%) of 40 persons with cryptococcal meningitis.38 Thus, improved outcomes are possible.
Mortality in high-income versus low and middle-income countriesAll countries in Latin America are classified as low or middle-income. The differences in outcome between Latin America and high-income countries have several potential explanations.
First, late testers and late presenters with HIV infection are more frequent in Latin America. Countries from Latin America present heterogeneous public health system and population showing substantial socioeconomic disparities. In this scenario, the human immunodeficiency virus (HIV) epidemic and opportunistic infections affect predominantly people living in poverty with limited access to timely and formal medical care.13 A cross-sectional analysis performed of 6047 HIV-infected HAART-naive patients at six sites in Latin America and the Caribbean reported 55% of late testers and 45% of late presenters, as defined as presenting with CD4<200 cells/μL.39 Another multi-cohort study with 5152 HIV-infected HAART-naïve patients at seven sites in Latin America and the Caribbean reported 76% with late HIV diagnoses.40 This prevalence is nearly twice as high as that observed in high-income settings (for example, 15-38% in Europe)41 and confirm the highly prevalent rate of late presenters and consequently late HAART initiation in Latin America. Consistently, low CD4 count at HAART initiation and more advanced disease constitutes strong predictors of mortality in the first year of HAART.40
Second, cryptococcal meningitis usually reveals HIV-infection in most settings.8,38,42 However, an additional scenario reported in Latin America is that a significant proportion of patients with cryptococcal meningitis are aware of their HIV-status prior to admission (with or without prior HAART use).24,25,27 This finding suggests continued missed opportunities to initiate or maintain HAART with persistent barriers to adherence and retention-in-care of a subset of patients.
Third, severe immunosuppression (also frequent in high-income countries) but particularly concomitant anemia, malnutrition, and severe cryptococcal meningitis are common in Latin America.24 The delay in presentation with diagnosis only when cryptococcal meningitis is advanced is common.24,43 This is evident by the increased proportions of patients presenting with neurologic complications in resource-limited setting.42,44
Fourth, rapid diagnosis is paramount to optimizing survival; however, diagnosis is difficult when optimum laboratory support is unavailable in most Latin American facilities. Although larger urban centers have a reasonable laboratory infrastructure, the availability of a timely diagnosis is highly variable by country and within country throughout Latin America.
Fifth, optimal medical management is not frequently utilized. Combination antifungal induction therapy and utilization of adequate measures to control of ICP are heterogeneous in routine practice. Combination antifungal therapy using amphotericin B plus a second adjunctive agent (e.g. 5-FC or high-dose fluconazole 800-1200mg/day in divided doses) has been associated with improved early fungicidal activity and with less mycological treatment failure.37,45–47 Early fungal clearance from the cerebrospinal fluid (CSF) is associated with 2-week and 10-week survival.29 Additionally, ICP management is likely suboptimal in routine practice. Even in the United States of America, frequent major deviation from optimal care with respect to increased ICP management was reported in a retrospective study of routine care from two tertiary care facilities in Washington, D.C.48 While no randomized controlled trial has been performed to validate ICP management, cross-comparison of studies using similar antifungal treatment regimens suggest there likely is 20–25% improvement in survival with aggressive ICP control. ICP management should be viewed as an integral component of cryptococcosis treatment.
Prognostic factors associated with mortalitySeveral risk factors for treatment failure or mortality have been reported elsewhere for AIDS-associated cryptococcal meningitis.24,29,37,49,50 These risk factors are likely relatively universal and applicable in Latin America. The major risk factors for mortality include: fungal burden (i.e. assessable by quantitative microscopy, quantitative cultures, and/or cryptococcal antigen titers), rate of fungal clearance, altered mental status, paucity of CSF WBC pleocytosis, abnormal brain imaging, elevated ICP at admission (which is uncontrolled), persistently elevated ICP, disseminated infection, and duration of antecedent symptoms.
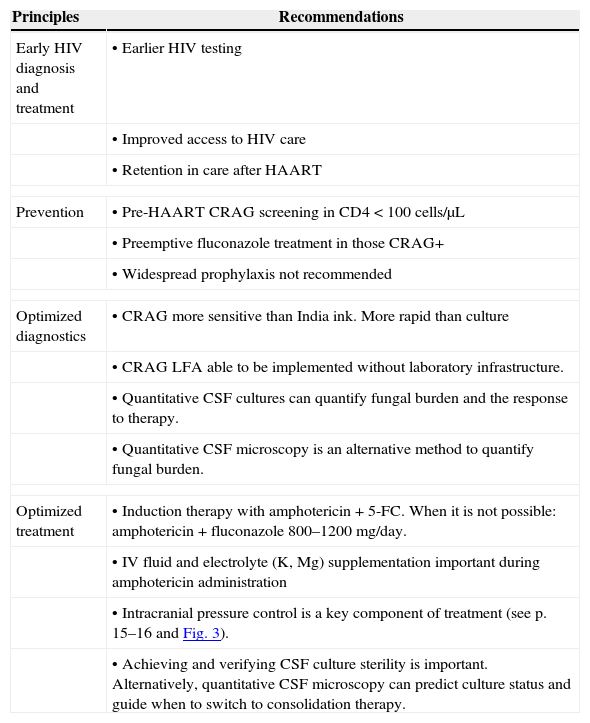
Strategies to reduce mortality and morbidity due to AIDS-related cryptococcal meningitisLawn et al. reviewed a number of strategies to reduce early mortality and morbidity during HAART.51 A similar approach can be taken to reduce the mortality and morbidity due to cryptococcal meningitis in Latin America. Potential key points to reduce mortality and morbidity are shown in Table 1. The strategies include: (1) early HIV diagnosis and treatment; (2) screening and preemptive treatment for subclinical cryptococcosis; (3) optimized diagnosis of cryptococcal meningitis; and (4) optimized treatment of cryptococcal meningitis.
Key recommendations to reduce mortality and morbidity due to AIDS-related cryptococcal meningitis.
| Principles | Recommendations |
|---|---|
| Early HIV diagnosis and treatment | • Earlier HIV testing |
| • Improved access to HIV care | |
| • Retention in care after HAART | |
| Prevention | • Pre-HAART CRAG screening in CD4<100cells/μL |
| • Preemptive fluconazole treatment in those CRAG+ | |
| • Widespread prophylaxis not recommended | |
| Optimized diagnostics | • CRAG more sensitive than India ink. More rapid than culture |
| • CRAG LFA able to be implemented without laboratory infrastructure. | |
| • Quantitative CSF cultures can quantify fungal burden and the response to therapy. | |
| • Quantitative CSF microscopy is an alternative method to quantify fungal burden. | |
| Optimized treatment | • Induction therapy with amphotericin+5-FC. When it is not possible: amphotericin+fluconazole 800–1200mg/day. |
| • IV fluid and electrolyte (K, Mg) supplementation important during amphotericin administration | |
| • Intracranial pressure control is a key component of treatment (see p. 15–16 and Fig. 3). | |
| • Achieving and verifying CSF culture sterility is important. Alternatively, quantitative CSF microscopy can predict culture status and guide when to switch to consolidation therapy. | |
CRAG, cryptococcal antigen; LFA, lateral flow assay; 5-FC, 5-flucytosine; IV, intravenous; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy.
Late HIV presenters have an increased risk of mortality and morbidity, compared with patients who present early.41 Thus, earlier HIV diagnosis and treatment are the most important and cost-effective preventive strategies to reduce the incidence and high mortality associated with cryptococcal meningitis.43 In Latin America, nearly 42% of people eligible for treatment were able to access HAART in 2009.52 Yet, continuation of HAART and avoiding HAART discontinuations are of major relevance. Reinforcing public health systems at the national and local level is necessary to obtain these challenging objectives in Latin America. Special focus must be offered to marginalized populations (e.g. commercial sex workers, men who have sex with men, injection drug users, and prisoners).
Pre-antiretroviral therapy careLinkage of testing to HIV care (engagement, counseling, monitoring, and support) is a key component of timely HAART initiation in order to avoid opportunistic infections, such as cryptococcal meningitis. Scaling up of infrastructure to attend HIV-infected patients who are not yet eligible for HAART is observed in Latin America but is highly dependent on the particular public health system. Lost to HIV-care and death is common prior to HAART initiation in low income-countries and minimizing the delays within the formal health system are essential.
Improving retention in care after HAART initiationAntiretroviral therapy programs in low-income settings often have retention-in-care of ∼60% of their patients at the end of two years,53 with loss to follow-up as the major cause of attrition, followed by death.53,54 However, a recent study performed in rural Rwanda demonstrates that comprehensive programs can have high retention rates (>90%) after two years of care.55 Reasons for the high losses to follow-up include transportation costs, food insecurity, user fees, drug stock-outs, toxicities, pill burden, comorbidities, and psychosocial reason like disclosure, stigma, and treatment fatigue.53,55 There is a need to identify innovative methods to sustain the provision of long-term care for patients receiving HIV care. For example, to decentralize the services by transferring patients initiating HIV care at tertiary centers (after stabilization) to primary care facilities closer to patients’ residence with inclusion of community-based approaches to HIV treatment. Improving retention-in-care remains a worldwide challenge.
Access to care after HAART initiationAlthough HAART treatment programs in low-income countries have similar efficacy rates to those reported in high-income countries,56 mortality during the first months of treatment is higher in low-income countries.57 These differences can be only partly explained by the lower CD4 cell counts and more advanced clinical stage. Cryptococcal meningitis after introduction of HAART (related to immune reconstitution or in patients with adherence problems) is a current challenge considering that access to prophylaxis, diagnosis facilities, and effective treatment is often limited in most low-income countries,57 including several from Latin America. Thus, key strategies to address higher mortality after HAART initiation in low-income countries include both earlier HIV diagnosis and HAART initiation, and improving access to and management of life-threatening opportunistic infections, especially cryptococcal meningitis.58
Screening and preemptive treatment for subclinical cryptococcosisSeveral studies performed in Africa have reported the prevalence of detectable serum cryptococcal antigenemia between 2-12% in patients with CD4<100cells/μL entering into HIV care. A positive serum cryptococcal antigen (CRAG+) when starting HAART predicts the development of cryptococcal meningitis, particularly when the CRAG titer is >1:8.59–61 Thus, the use of routine serum or plasma CRAG screening in asymptomatic HAART-naïve adults with CD4<100cells/μL, followed by preemptive fluconazole therapy can reduce the development of cryptococcal meningitis and improve survival. The World Health Organization (WHO) recommended preemptive treatment for subclinical CRAG+ is fluconazole 400mg twice daily for two weeks followed by 400mg daily for eight weeks.43 This screen and treat strategy is highly cost-effective, and is recommended by the WHO in locales where the prevalence of cryptococcal antigenemia is >3%.43,59 Although this strategy has not been formally evaluated in Latin America, epidemiological data suggest its potential benefit and cost-savings.1,25,61 Nevertheless, in centers where lumbar puncture in readily available, asymptomatic patients with CRAG+ identified on screening, should likely have an lumbar puncture, to exclude active disease. The use of point-of-care CRAG lateral flow assay (LFA) tests seems to be ideal for screening for cryptococcosis, but there are no published data as yet.
Prophylaxis for cryptococcal meningitisThe routine use of antifungal primary prophylaxis for cryptococcal meningitis in HIV-infected patients with CD4 count <100cells(μL is not recommended prior to HAART in those who are CRAG-negative or where CRAG testing is unavailable, unless a prolonged delay in HAART initiation is likely.43 While effective, primary prophylaxis is not recommended as this approach is less cost-effective than CRAG screening coupled with preemptive treatment.
Optimized diagnosis of cryptococcal meningitisCurrently, cryptococcal meningitis diagnostics focus on: direct CSF examination by microscopy (India ink), CSF culture, or antigen detection using latex agglutination (LA) or enzyme immunoassay (EIA). The CRAG latex assay is a relatively simple test that affords high sensitivity and specificity, but its broad use in Latin American is limited by the costs and by the need for laboratory infrastructure. Recently, an immunochromatographic CRAG lateral-flow immunoassay (LFA), has been developed as a point-of-care test for diagnosis of cryptococcosis (Immy Inc., Norman, Oklahoma, USA). This LFA test is highly sensitive, inexpensive, and can be performed by untrained personnel in a point-of-care setting with results in ≤10min.62 In July 2011, the U.S. Food and Drug Administration (FDA) approved the CRAG LFA for use in serum and in April 2012 for use in CSF. A recent study confirmed the excellent concordance of LFA with traditional latex agglutination in both plasma and CSF samples.63 Countries should implement widespread and reliable access to rapid diagnosis by either cryptococcal antigen test.24 LFA cost is US$ 2 for low income countries and US$ 5 for middle and high income countries. The LFA has greater applicability to the region as the assay can be implemented without laboratory infrastructure or refrigeration of reagents. Local studies are expected in Latin America.
Response to treatment of cryptococcal meningitis currently is monitored by the use of CSF cultures in most reference centers from Latin America. Quantitative cultures can identify fungal burden and the rate of clearance;28,29,49 however, any culture results have an intrinsic delay in reporting, and quantitative cultures are not used in routine practice in Latin America. Thus, rapid and simple methods to estimate fungal burden remain clinically useful worldwide. Recently, we reported quantitative CSF microscopy counting cryptococcal yeasts per mcL of CSF was a useful intervention to predict CSF culture status and outcome. A baseline fungal burden of ≥10 yeasts/μL of CSF by quantitative CSF microscopy was associated with a 15-fold higher odds of failing sterilize the CSF by 7–14 days (p<0.001). Similarly, at 7–14 days, >10yeasts/μL of CSF was associated with continued positive CSF cultures in 98% vs. 36% when <10yeasts/μL of CSF (p<0.001).24 Thus, this relatively simple tool could be used as a guide for when to switch from intensive induction amphotericin therapy to consolidation fluconazole monotherapy.
Optimized treatment of cryptococcal meningitisThe importance of rapid CSF fungal clearance is supported by an association between two-week culture status and 10-week clinical outcome.49 The current recommended standard of care is the use of combination antifungal therapy during at least a 2-week induction phase.43,64 In Latin America the most frequent treatment is monotherapy with amphotericin B, as 5-FC is usually unavailable, and combined therapy with amphotericin B and fluconazole is as yet infrequent.
For the two week induction treatment phase, a regimen containing amphotericin B combined with 5-FC is recommended.43,64 Alternatively, the combination of amphotericin B plus high doses of fluconazole (800–1200mg/day in divided doses) is recommended where 5-FC is unavailable.43,64 This combination is supported by limited evidence in two phase II clinical trials that showed a marginally superior rate of CSF clearance with amphotericin B plus fluconazole when compared with amphotericin alone,65 and similar CSF early fungicidal activity with amphotericin plus fluconazole 1200mg/day as with amphotericin plus 5-FC.47 In addition, in an open-label randomized clinical trial, amphotericin combined with fluconazole 800mg/day for 2-weeks results in superior but non-statistically different outcomes as compared to treatment with 4-weeks of amphotericin monotherapy.66 When giving fluconazole at doses above ≥800mg/day, we recommend to divide the doses to reduce nausea.20 With induction therapy with amphotericin B (0.7–1.0mg/kg/day) and fluconazole 800mg/day, the expected average time to CSF sterilization is 13.5 days based on the average early fungicidal activity and median fungal burden present in Sub-Sahara African studies.28–30,42,47,49,67,68
After induction therapy, the next phase is eight weeks of consolidation treatment with fluconazole monotherapy at doses of 400–800mg/day. The differences in dosing is related to the activity of fluconazole, whereby doses of 400mg/day are fungistatic whereas doses ≥800mg/day are fungicidal.30,67 In situations where the 2-week culture status is as yet unknown, we recommend starting consolidation therapy at 800mg/day of fluconazole (in divided doses) until the CSF culture is known to be sterile and until HAART has been initiated. Once the CSF culture is known to be sterile, consolidation therapy with 400mg/day of fluconazole is likely sufficient. Alternatively, quantitative CSF microscopy could be used as a guide for when to switch from intensive induction amphotericin therapy to consolidation fluconazole monotherapy (400–800mg/day).24 After completion of 8-weeks of consolidation therapy, secondary prophylaxis as maintenance treatment phase with fluconazole 200mg/day is recommended for at least one year and until the CD4>200cells/μL.43,64
Recently, a WHO guideline recommended that patients with amphotericin B-containing regimens should receive a minimal package of toxicity prevention, monitoring, and management to minimize serious complications, particularly hypokalemia and nephrotoxicity.43 This package of care includes pre-hydration with one L of normal saline coupled with oral electrolyte supplementation with 40mEq daily of potassium and 16mEq daily of magnesium. Although hydration prior amphotericin infusion is a common practice in Latin America, the “preemptive” supplementation of potassium is not used in most centers and could be incorporated in routine clinical practice. This electrolyte supplementation is especially important in settings with limited laboratory monitoring and/or delays in reporting of laboratory results. Liposomal and lipid formulations of amphotericin are not available in most public centers; however, when pre-hydration is used, severe acute renal failure is relatively uncommon (<5%) in most patients with cryptococcal meningitis. However, when acute renal failure does occur or its risk is high, when possible, liposomal/lipid formulations of amphotericin B should be utilized.43 Where lipid formulations are unavailable, alternative options are early discontinuation of amphotericin coupled with high dose fluconazole 1200mg/day for 4-weeks.67,68 This supplemental package of care is an important addition to routine cryptococcal care to minimize amphotericin toxicity.
In Latin America, there are other unique considerations, which are different than most countries from Sub-Saharan Africa. In Latin America, cerebral toxoplasmosis is the primary cause of acute neurologic symptoms among HIV-infected patients. Cranial nerve VI palsy is common with increased intracranial pressure due to cryptococcal meningitis; however, other cranial nerve palsies very rare.28,69 Therefore, central nervous system imaging should be performed before lumbar puncture, particularly when there are localizing focal neurologic deficits present.
Although persons with AIDS-related cryptococcal meningitis present with fewer cryptococcomas than non-HIV-infected persons, other abnormal lesions (e.g. pseudocysts or dilated Virchow-Robin spaces) are frequent,24,50 particularly with magnetic resonance imaging (MRI), a more sensitive technique than computed tomography (CT).50 The presence of these abnormal lesions is associated with worse prognosis.50 Disseminated cryptococcosis throughout the body and brain is common, even when not clinically apparent. One histopathology study from Brazil reported a high frequency of encephalitic involvement and focal brain lesions among patients with cryptococcal meningitis.70 In many cases, dissemination is often subclinical. Current recommendations suggest to prolong the induction treatment phase in patients with cryptococcomas;64 however, there is little evidence-based data to guide the treatment of other forms of parenchymal involvement. Taken together, this information and our unpublished data of cases observed in São Paulo, we suggest that these cases must be treated similarly to cryptococcomas and clinical, microbiologic, and radiologic follow-up are necessary for optimum management.
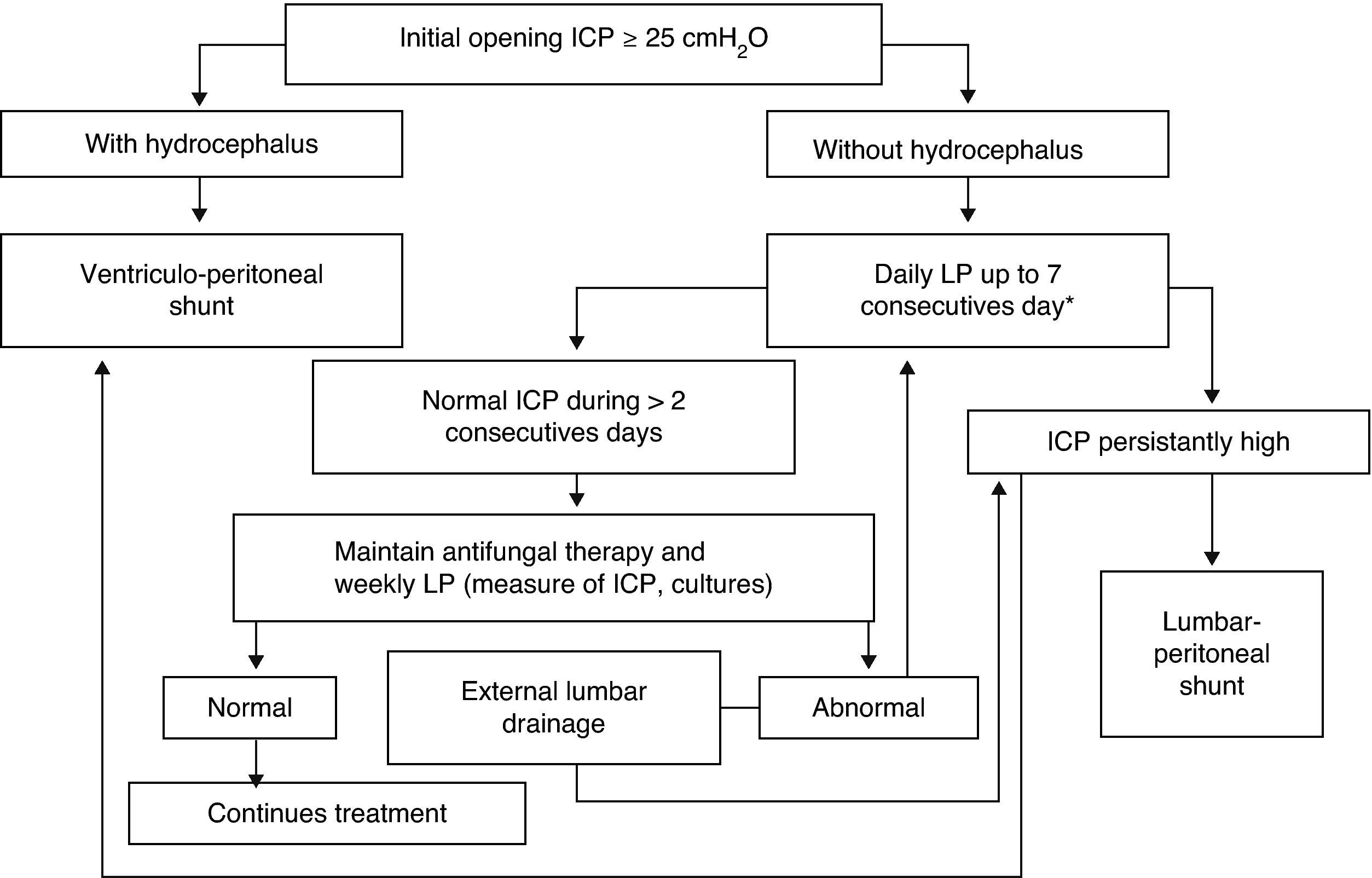
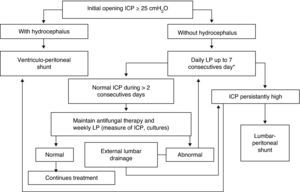
Increased ICP is frequent with cryptococcal meningitis, and ICP management is an essential component of medical care. Elevated ICP (≥25cm H2O) is associated with reduced short-term survival and impaired treatment response.71 For these reasons, systematic and aggressive measures to control elevated ICP are critical and not to overlooked. Based on published recommendations,9,43,64,71–73 the cornerstones of management are summarized as follows. First, measure CSF opening pressure at baseline with a manometer (in our setting always after imaging); if the pressure is ≥25cm H2O of CSF and/or there are symptoms of increased ICP, drain ∼20mL of CSF. Removal of more fluid than necessary has the potential risk of a post lumbar puncture headache, whereas lack of ICP control has a risk of death. Second, if there is persistent value ≥25cm H2O or symptoms, repeat therapeutic lumbar punctures daily until the CSF pressure and symptoms have been stabilized for >2 days. Most patients require 2–3 lumbar punctures for adequate control of ICP. One lumbar puncture is rarely sufficient (≤25% of patients) to control the ICP. When in doubt (i.e. no manometer), we recommend to err on the side of removal of too much CSF than too little, with 15–20mL being an average amount removed in prospective studies in Africa.28,69 Third, CSF examination should be repeated to confirm a therapeutic response (negative CSF culture by 14 days). Fourth, in the presence of persistently elevated ICP, consider temporary external lumbar drainage, or alternatively, external ventricular drainage, for persons who require repeated daily lumbar puncture. Evaluate permanent shunt (lumbar-peritoneal or, alternatively, ventricular-peritoneal) if the patient is receiving or has received appropriate antifungal therapy and if the standard methods of repeated therapeutic lumbar punctures have failed to control the ICP. Ineffective method of ICP control include: mannitol, acetazolamide, and corticosteroids which are not supported nor recommended by the available evidence.64Fig. 3 displays the algorithm for the management of elevated ICP of our institution.
Algorithm of management of intracranial pressure in AIDS-related cryptococcal meningitis. Emilio Ribas Institute of Infectious Diseases, São Paulo, Brazil. LP, lumbar puncture; ICP, intracranial pressure; CT, computerized tomography. * Alternatively, a temporary external lumbar drainage can be placed.
The optimal time to initiate HAART in AIDS-related cryptococcal meningitis is undefined. Although some studies showed benefits with earlier HAART initiation,74,75 only few cases of cryptococcal meningitis were included in one of the trials.74 By contrast, a small randomized clinical trial compared early HAART (≤72h after diagnosis) versus deferred HAART (≥10 weeks of treatment). In this study performed in Zimbabwe, early initiation of HAART (stavudine, lamivudine, and nevirapine) results in increased mortality when used with fluconazole 800mg/day monotherapy as induction therapy.76 Despite several limitations,77 this study suggests that very early initiation may be harmful. Thus, HAART initiation should be deferred until there is evidence of a sustained clinical response to antifungal therapy. The WHO recommended timing of ART initiation is after 2–4 weeks of induction and consolidation treatment with amphotericin B-containing regimens combined with flucytosine or fluconazole.43 Sterilization of the CSF prior to HAART initiation and before changing to 400mg of fluconazole consolidation therapy should be the goal. More recently, a phase IV randomized clinical trial in Uganda and South Africa investigating the optimal time to initiate HAART (efavirenz-based) after amphotericin-based treatment for cryptococcal meningitis (clinicaltrials.gov NCT01075152) was halted early after 177 participants were enrolled. Data and safety monitoring board (DSMB) found a statistically higher mortality among the participants randomized to early HAART (median 8 days) compared with delayed HAART (median 5 weeks). This trial confirms that early HAART in cryptococcal meningitis does not have an overwhelming survival benefit and very well may be harmful.78 Based on our present knowledge, HAART initiation should be delayed until completion of induction therapy with an objective to start HAART within 4–5 weeks. Further delay beyond this timeframe is likely unwarranted. Linkage to outpatient HIV care remains a critical consideration for a delayed HAART strategy.79–81
Immune reconstitution inflammatory syndromeRapid restoration of immune function after starting HAART leads to enhanced cell-mediated responses to live or dead organisms or shed Cryptococcus antigen that may present as either (1) unmasking of subclinical infection in those who are CRAG+ pre-HAART; or (2) recurrence of symptoms and signs of previously identified and treated infection.82 Unmasking IRIS can be prevented by pre-HAART CRAG screening and preemptive therapy. Unmasking cryptococcal disease is extremely rare in persons who are pre-HAART CRAG-negative. In persons with pre-HAART diagnosed cryptococcal meningitis who respond to antifungal therapy and then start HAART, paradoxical IRIS can occur with recurrence of meningitis symptoms or new non-CNS manifestations, such as lymphadenopathy or pneumonitis.83 The average incidence of paradoxical IRIS is 16% in one meta-analysis.84 In a Brazilian study this figure was 23%.85 Risk factors for IRIS-related cryptococcosis include a high baseline fungal burden (i.e. fungemia and high serum cryptococcal antigen), failure to sterilize the CSF by 2-weeks, less initial CSF inflammation, lack of pro-inflammatory cytokine serum responses, low CD4 count (i.e. CD4<50cells/μL), and more rapid immune reconstitution.69,76,77,79–82,85,86 Symptoms usually develop early, at a median of 6-10 weeks after HAART initiation.82,85,87 Main neurologic manifestations of cryptococcal IRIS reported in the literature include recurrent meningitis with or without increased ICP in the setting of sterile cultures and/or cryptococcomas.82,83,85 CSF cultures are consistently negative in paradoxical CM-IRIS.82
Although the treatment of cryptococcal-related paradoxical IRIS is not completely understood, it is reasonable to administer systemic corticosteroids for severe life threatening manifestations.82,83,87 Furthermore, serial lumbar punctures are likely required to manage high ICP in these patients; however, discontinuation of HAART is usually unnecessary.82,83,85 Successful relief of symptoms by daily lumbar punctures was confirmed in a Brazilian case series.85 Similar approaches of aggressive ICP control in IRIS have been used in Thailand. For refractory paradoxical IRIS cases, anti-tumor necrosis factor (TNF) agents have been anecdotally used, including thalidomide and anti-TNF monoclonal antibodies.88 Mortality at six months may be similar or increased between IRIS and non-IRIS patients.69,82,87 In Latin America, only one Brazilian study about cryptococcal-related IRIS has been published.85 More regional studies about this challenging problem are necessary.
ConclusionsGlobally, Latin America represents the third region with most cases of AIDS-related cryptococcal meningitis. Despite relevant improvements during the HAART-era, this opportunistic infection remains frequent and causes an unacceptable high mortality in most countries. Urgent implementation of strategies both on the public health level and individual care level are necessary to reduce mortality and morbidity with consideration of the local specific particularities and the heterogeneity of Latin America.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Marcia Polon from the Division of Epidemiology Surveillance of the Centro de Referência em Treinamento em DST/AIDS from São Paulo State, Brazil, for the data about AIDS-related cryptococcal meningitis from Sao Paulo State. DRB receives support from the U.S. National Institute of Allergy and Infectious Diseases (K23AI073192).