COVID-19 can trigger different clinical presentations in distinct population groups, some of which are considered at higher risk of SARS-CoV-2 infection. Little is known about the susceptibility of certain populations to the infection.
ObjectivesWe aimed to determine the prevalence of COVID-19 among People Living With HIV/AIDS (PLWH) attending a tertiary public hospital in Salvador, Brazil, patients with active pulmonary tuberculosis and Hospital's Healthcare Workers (HCW), and to compare their SARS-CoV-2 antibody levels.
MethodsIn this observational study we included 2294 participants from June 9, 2020 to August 10, 2021. IgG SARS-CoV-2 antibodies from all participants (275 PLWH, 42 with active tuberculosis and 1977 healthcare workers) were measured. Prevalence of COVID-19 and antibodies indexes were compared across groups.
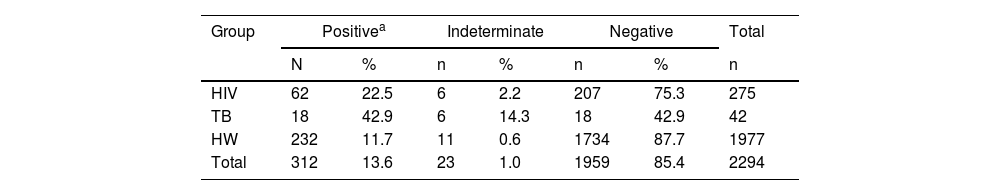
ResultsWe detected a higher prevalence of COVID-19 in patients with active tuberculosis (42.9%) than in PLWH (22.5%) or HCW (11.7%). Previously vaccinated participants with a COVID-19 history had median higher IgG antibody indexes (8.2; IQR: 5.5‒10) than those vaccinated who did not have COVID-19 until the time of this study (4.1; IQR: 1.6‒6.2, p < 0.001).
ConclusionPrevalence of previous SARS-CoV-2 infection was higher among tuberculosis patients than that found in HCW and PLWH, but antibodies levels were similar across groups.
The COVID-19 pandemic affected over 600 million people and caused more than 6 million deaths globally.1 Death rates were higher for older individuals and people with underlying diseases such as hypertension, diabetes mellitus and coronary heart disease than for the general population. HIV and tuberculosis, two of the most important infectious diseases worldwide, were classified by Centers for disease Control and Prevention (CDC) as risk factors for severe COVID-19.2
Patients living with HIV on suboptimal therapy, or persistent viral replication for different reasons can present a chronic inflammatory state and a persistent immune activation. 3 Mycobacterium tuberculosis and SARS-CoV-2 share the lungs as the main site of infection, and also the capacity of promoting an exacerbated immune response.4 However, there is limited information on the serological response to the SARS-CoV-2 infection in these populations. It is not clear whether serological response to COVID-19 in ART controlled HIV infected individuals or patients with active tuberculosis is different from the general population. This study aimed to determine the prevalence of COVID-19 among People Living With HIV/AIDS (PLWH), patients with active tuberculosis and to compare the antibody index rates in such populations and in a control group formed by healthcare workers (Table 1). Participants aged 18-years or older, with an earlier diagnosis of HIV, followed at the Infectious Diseases Outpatient Clinic of Professor Edgard Santos University Hospital (HUPES) who attended for medical visits, testing or drug withdrawal, and patients with active pulmonary tuberculosis without a history of co-infection with HIV who attended a referral center in Salvador, Brazil (the Brazilian Institute for Thorax Investigation ‒ IBIT) for medical follow-up were invited to join this cohort. After consent, the team applied a questionnaire on clinical and sociodemographic data to PLHW and tuberculosis patients, followed by blood samples collection. Data from healthcare workers employed at HUPES were obtained from a previous study that contained the same information, to form a control group.5 Enrollment of participants took place between June 9, 2020 and August 10, 2021, a period before vaccination against COVID-19 was available, between the first and second waves of COVID-19 pandemic in Brazil. After a median of 280 days since the inclusion, between February 18, 2021 and November 11, 2021, 163 study participants had a second blood collection, after receiving at least one vaccine dose. Of these, 45 were from the PLWH group and 118 from the HCW group.
Prevalence of COVID-19 by group of participants, in Salvador Brazil, 2020.
| Group | Positivea | Indeterminate | Negative | Total | |||
|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | n | |
| HIV | 62 | 22.5 | 6 | 2.2 | 207 | 75.3 | 275 |
| TB | 18 | 42.9 | 6 | 14.3 | 18 | 42.9 | 42 |
| HW | 232 | 11.7 | 11 | 0.6 | 1734 | 87.7 | 1977 |
| Total | 312 | 13.6 | 23 | 1.0 | 1959 | 85.4 | 2294 |
IgG SARS-CoV-2 antibodies from plasma samples were determined by enzyme immunoassay (ELISA) method. Anti-SARS-CoV-2 IgG kit (Euroimmun, Lübeck, Germany) for semiquantitative detection of antibodies against subunit 1 of the spike protein was used according to the manufacturer's recommendations. Results were given as indexes from sample absorbance/calibrator absorbance.
Statistical analyses were carried out using the IBM SPSS software. Categorical variables were given as percentages and continuous variables were reported as median and Interquartile Ranges (IQR). Kruskal-Wallis test was used to compare the three groups in this study, and Pearson's chi-square test was used to determine whether frequencies were different. Statistical significance was defined as p-value <0.05. Cases with an indeterminate result were excluded from calculation of median antibody index.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Maternidade Climério de Oliveira Hospital, from Universidade Federal da Bahia (protocol code 4.290.254, April 21, 2020). Informed consent was obtained from all subjects involved in the study.
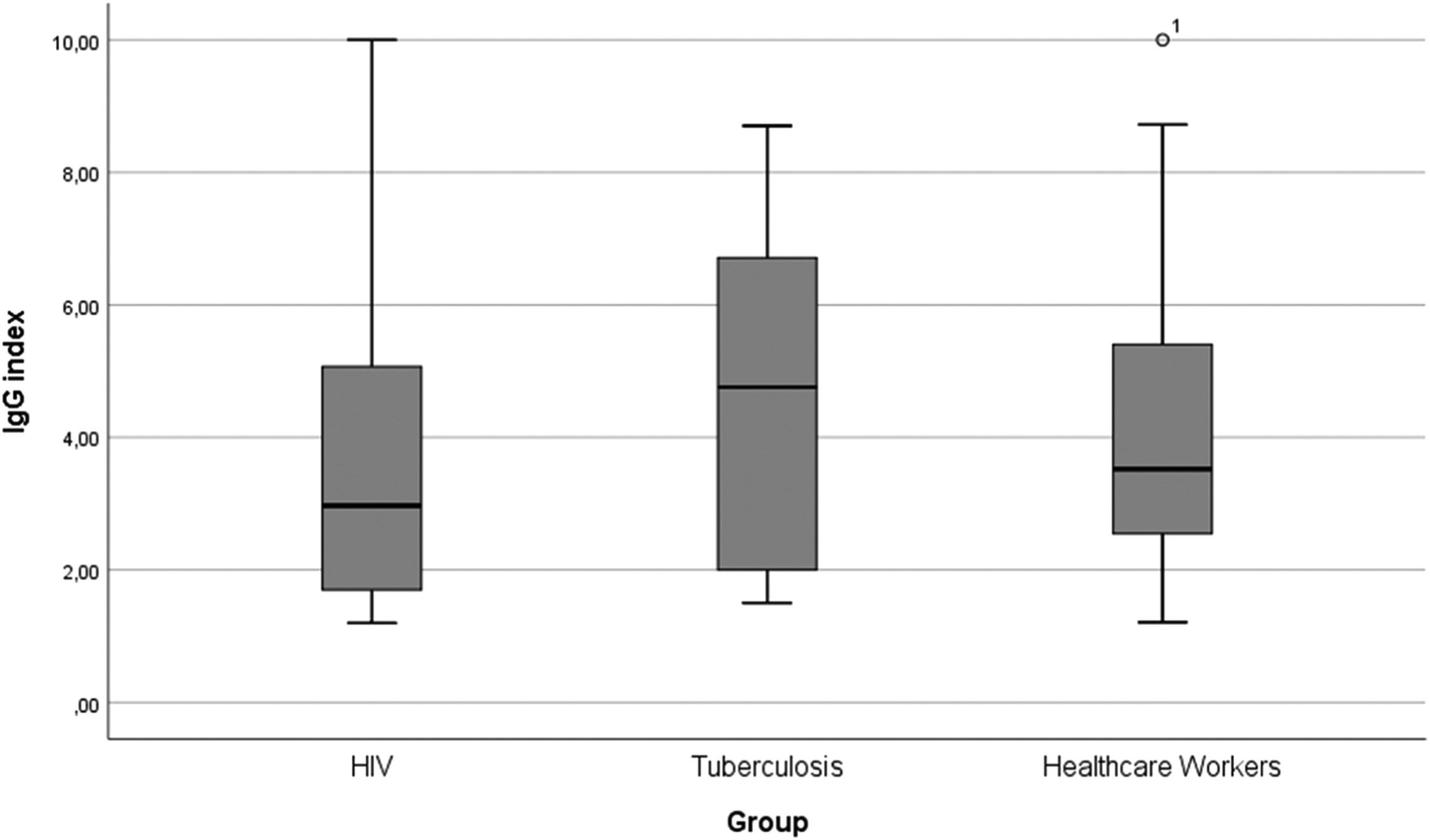
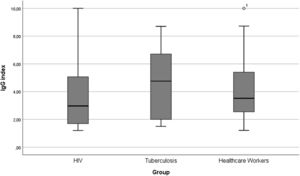
The study population included 2294 participants, 275 People Living With HIV (PLWH group), 42 with active pulmonary Tuberculosis (TB group) and 1977 in Healthcare Workers (HCW group). Median age of participants in PLWH group was 47.0 years (IQR: 37.5‒54.5), 38.7-years (26.7‒48.5) among participants with active tuberculosis and 39.7 (33.0‒47.0) in HCW group. Most participants in PLWH and TB groups were male (69.8% and 69.0%, respectively), while the frequency of women was higher among HCW (71.7%). Median Body Mass Index (BMI) was 24.49 (22.2‒28.0) in the PLWH group, 21.9 (20.0‒27.2) in the TB group and 25.2 (22.9‒28.5) in the HCW group. In the PLWH group, 5.8% of participants had diabetes mellitus, while 2.4% from TB group and 2.3% from HCW had the condition. Median IgG index for antibodies against SARS-CoV-2 in PLWH group was 3.0 (IQR: 1.7‒5.1), 4.8 (IQR: 2.0‒6.8) in TB group and 3.6 (IQR: 2.5‒5.4) in HCW group (Fig. 1). The difference between IgG indexes within the three groups was not statistically significant.
Vaccinated individuals with a COVID history had higher IgG indexes than those vaccinated participants who did not get SARS-CoV-2 infection (8.2 [IQR: 5.5‒10] versus 4.1 [IQR: 1.6‒6.2]; p<0.001). The number of days for the second blood collection was similar (p = 0.13) across these groups. Twenty-three cases had an indeterminate result and so were excluded from calculation of median antibody index.
Some risk factors of the total sample of participants were assessed and no significant difference was found for gender (p = 0.21), BMI (p = 0.78) and diabetes mellitus (p = 0.32). In average, participants who returned for the second blood collection were 3.4-years older than the initial group.
The highest frequency of previous COVID-19 was observed among participants with active tuberculosis, followed by PLWH group, while HCW showed the lowest frequency of SARS-CoV-2 IgG antibodies. Considering that we cannot determine which of the infections, tuberculosis or COVID-19, occurred first, this interaction may be approached from a dual perspective. An observational case-control study of COVID-19 cases from China suggest that latent or active tuberculosis may increase susceptibility to SARS-CoV-2 infection, and that COVID-19 disease progression may be more rapid and severe in patients with tuberculosis.6 Petrone et al. found a reduced production of anti-SARS-CoV-2 antibodies in a COVID-19-TB coinfection setting.7 On the other hand, the immunosuppressive characteristics of COVID-19 could potentially play a role in the reactivation of latent tuberculosis.8 A systematic review of case reports revealed that TB reactivation or new TB infection became a common phenomenon in individuals who recovered from COVID-19.9 Further investigations are necessary to better understand this complex interaction.
We observed a higher frequency of positivity for COVID-19 among PLWH compared to that found in other countries.10,11 Although some authors point to a higher susceptibility of HIV-infected patients to COVID-19,12,13 this is still a controversial issue. Previous reports detected a similar risk of infection to that found in general population, which suggest HIV infection is not an independent risk factor for COVID-19 infection.14,10 In a study conducted at a University Hospital in Southern Spain after one year of the pandemic, researchers found a lower incidence of COVID-19 among PLWH compared to the general population treated in the same hospital area. 15 Most of patients included in our study were on stable ART and therefore, had median CD4 count within a normal range (658 cells/μL – IQR: 446.5‒872.0). A study conducted in France revealed a lower rate of seroconversion in PLWH with CD4 counts less than 500 cells/μ, and even lower in PLWH with less than 200 cells/μ, compared with those with CD4+ T-cell counts greater than 500 cells/μL.16
Despite healthcare professionals are inherently exposed to a higher risk of infection due to the nature of their work, they presented with a lower incidence of COVID-19 infection compared to other groups. This can likely be attributed to a greater adherence to recommended preventive measures, in contrast to other populational groups. Our results showed lower prevalence of infection among HCW than previously reported. 17 In a previous work we showed that in our setting the main risk factor associated with COVID-19 among healthcare workers were lower education and lower socioeconomic profile, regardless exposure. 5
In this study the prevalence of COVID-19 was estimated before the start of vaccination for COVID-19, in a period that comprised the first and second waves of the pandemic in Brazil. Conducting studies at diverse epidemiological intervals, variety on diagnostic methods, development of studies at distinct care centers profiles and the variable effectiveness of public policies at the national level should be considered as contributing factors to these differences on prevalence.
Median basal antibodies indexes among the three groups were compared, and no difference was identified, revealing similar immunological responses. Due to the small sample size of the TB group, medians of IgG antibodies in the other two groups were compared, and the difference between them remained not statistically significant. A few serological results were indeterminate and were excluded from analysis. These occurrences might be attributed to the possibility of ongoing seroconversion at the time of blood collection. We were unable to find any previously published report comparing antibodies index for these specific populations.
Higher IgG indexes detected among vaccinated participants who also had COVID-19 versus those vaccinated who did not get the infection confirms that a previous infection followed by a vaccine dose promotes a booster effect. This finding is consistent with data from Tan et al., 18 who found that cases who had received at least one COVID-19 vaccine dose had 24% lower risk of transmitting infection than unvaccinated cases, whereas having both prior vaccination and SARS-CoV-2 infection was associated with a 41% reduction on risk of transmission. Bates et al. showed that SARS-CoV-2 infection before or after vaccination significantly increase neutralizing antibody response compared to two doses of vaccine alone.19
Our study has some limitations. Only 17% of HIV patients and 6% of healthcare workers returned for the second blood sampling due to country lockdown or because they had not yet been vaccinated. However, there was no significant difference between the group that returned for the second blood collection and the initial group, considering the variables gender, BMI, and having diabetes. The age difference found between the two groups can be explained by the fact that older people were among the first to receive COVID-19 vaccine in Brazil.
Our study shows a higher prevalence of COVID-19 among tuberculosis patients, than that observed among PLWH and HCW. Interaction between COVID-19 and tuberculosis may rise a bidirectional discussion, considering that clinical evidence suggests both, increased susceptibility to tuberculosis due to SARS-CoV-2 infection and the possibility of reactivation of latent tuberculosis in the presence of COVID-19. Further investigations are needed to better understand this important clinical challenge. The susceptibility of PLWH to COVID-19 remains uncertain, but the available evidence suggest that PLWH on stable antiretroviral therapy are not at greater risk of contracting COVID-19 than the general population. We demonstrate that IgG antibody indexes did not differ for the three studied groups, which suggest similar immunological responses. Our study confirms the booster effects of vaccination together with a previous COVID-19 infection on the production of antibodies against SARS-CoV-2 when compared to vaccination without previous COVID-19.