The Taenia solium cysticercosis affects millions of people worldwide and is considered a public health problem, especially in developing countries. The diagnosis of neurocysticercosis is complex and involves the analysis of epidemiological, clinical, neuroimaging, and immunological host data. Neurocysticercosis is endemic in Brazil, and is related to the cause of death mainly in the Southeast, South, and Central-West regions. The objective of this study was to determine the seroprevalence of cysticercosis in inhabitants of the city of Jataí, Goiás, in the Central-West region of Brazil from April to August 2012. A total of 529 serum samples were analyzed by enzyme-linked immunosorbent assay for detecting IgG antibodies against T. solium larvae, and Western blotting was used for confirming the diagnosis through the recognition of at least two specific peptides from their serum antibodies. The 351/529 (66.3%) reactive samples were analyzed by enzyme-linked immunosorbent assay and Western blotting confirmed the diagnosis in 73 samples that recognized at least two of the following peptides specific IgG antibodies for cysticercosis: 18, 24, 28–32, 39–42, 47–52, 64–68, and 70kDa. The seroprevalence of cysticercosis was 13.8% (95% CI 5.9–21.7), demonstrating that the studied area is endemic to this disease.
The Taenia solium cysticercosis affects millions of people worldwide and is considered a public health problem, especially in developing countries.1 The T. solium life cycle involves both human and pig hosts. Cysticercosis occurs after ingestion of eggs of T. solium by fecal contamination of human host. Larval infection can be diagnosed in different tissues, such as skeletal muscle, subcutaneous and central nervous system tissue.2 Human neurocysticercosis (NCC) is the most severe form, because about five million cases of epilepsy worldwide are related to this parasitosis.2 NCC is a neglected tropical disease recognized by the World Health Organization since 2010; it is endemic in several countries in Latin America, sub-Saharan Africa and Asia, including India, most of Southeast Asia, and China.3 Agapejev4 conducted a critical analysis of Brazilian literature (1915–2002) which showed an NCC frequency of 1.5% in autopsies and 3.0% in clinical studies. In seroepidemiological studies the positivity of specific reactions was 2.3%. In Brazil, between 2000 and 2011, a total of 1829 deaths related to NCC were recorded, which represented 0.015% of all deaths. High-risk clusters of NCC-related mortality were evidenced in endemic areas in the Southeast, South and Central-West regions.5 The North and Northeast regions usually have no specific control programs; people from these areas have limited access to health services, and the living conditions are poor.4,5
This zoonosis is directly related to risk factors such as disordered urbanization, precarious conditions of basic and hygienic sanitation, close contact with pigs, and poor health surveillance present in the regions where the human infection is endemic, as well as cultural behavior and internal migration of people from rural areas to urban centers.5,6 Swine cysticercosis is not well recorded in Brazil due to clandestine slaughter of pigs, along with limited inspection and sanitary control.7 Notification of diagnosed cases of NCC is not considered compulsory in Brazil, although it is recommended by the Ministry of Health. Only the South and Southeast regions of the country use this strategy to investigate infection cases for prevention programs with increased coverage and access to diagnosis through neuroimaging tests, along with clinical and surgical treatment.4 Prevention and control of T. solium transmission should be a priority as intervention can reduce the substantial healthcare and economic burdens due to both NCC and taeniasis.8
The objective of this study was to determine the seroprevalence of human cysticercosis in serum samples from inhabitants of the municipality of Jataí, Goiás state, in the Central-West region of Brazil.
The study was carried in the municipality of Jataí (17° 52′ 53″S, 51° 42′ 52″W), 330km from the capital of Goiânia, located in the southwest of the state of Goiás, Brazil. The municipality area of 7174km2 has an estimated population of 97,077 inhabitants.9 This study was approved by the Research Ethics Committee of the Federal University of Goiás (UFG), under protocol number 230/2011.
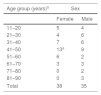
A total of 529 individuals (301 females and 228 males) participated in the study. All individuals older than 18 and representatives in the case of children (≥4 years old), who agreed to participate in the study, signed the informed consent. They were selected voluntarily from the waiting rooms of three clinical analysis laboratories (two private and one public) in the city under study. The participants consented to donate 2mL of serum remaining after the serological and/or biochemical tests that were requested by the doctors who took care of them. The serum samples were donated from April to August 2012, transported under refrigeration and stored at −20°C at the Immunology Laboratory of UFG Regional Jataí.
A total saline extract with 50 metacestodes of T. solium was prepared and the obtained extract was divided into aliquots of 200μL, identified and stored at −20°C until processing. Serum samples were submitted to the ELISA test in accordance with Barcelos et al.10 Some 200μg of the total saline extract protein was applied by 10×8cm gel and subjected to 12% polyacrylamide gel electrophoresis (SDS-PAGE) for separation of the peptides present in the antigenic extract and subsequent transfer to 0.45μm pore size nitrocellulose membranes. After transfer, the nitrocellulose membranes were cut into 3mm strips. The antigenic peptides recognized by IgG antibodies present in the reagent serum samples in the ELISA were analyzed by Western blotting (WB).10 The criterion for positivity in WB adopted was the recognition of at least two of the peptides, 18, 24, 28–32, 39–42, 47–52, 64–68, and 70kDa, in WB specific for cysticercosis.10 The results obtained in the ELISA and WB were analyzed using two-way ANOVA and Fisher's exact tests with p≤0.05 in the software GraphPad Prism version 5.0. The total saline extract of T. solium metacestodes was prepared for use in both tests and presented a protein concentration of 3000μg/mL.
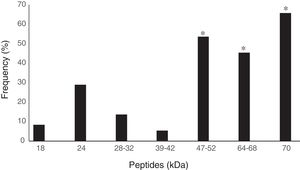
All 529 serum samples were analyzed by ELISA to detect IgG antibodies against cysticercosis and 351 (66.3%) samples were reactive. All reagent serum samples analyzed by ELISA were submitted to WB, resulting in 37 samples (10.5%) with no peptide recognition and 314 (89.5%) were reagents. Seventy-three of the 314 samples with two or more peptides were considered reagent according to the diagnostic criterion adopted in WB for cysticercosis10 (Table 1). Therefore, 13.8% (95% CI 5.9–21.7) of the 529 serum samples analyzed showed T. solium anti-metacestode antibodies in ELISA and recognition of specific peptides for NCC in WB. Of the 73 participants that showed specific band recognition in WB, 38 (52%) were female and 35 (48%) male. Positivity among females aged 41–50 years was significantly higher (p=0.0182) (Table 2). The specific peptides that were significantly more often recognized by the antibodies were 64–68 (45.2%), 47–52 (53.4%), and 70kDa (65.7%) peptides (p=0.01) (Fig. 1).
Frequency of peptide recognition by Western blotting test through IgG antibodies against the total saline extract of Taenia solium metacestodes in 314 serum samples from inhabitants of the municipality of Jataí, Goiás, from April to August 2012.
| Type and number of peptides recognized | Number of reagent samples | Recognition frequency (%) |
|---|---|---|
| Only non-specific | 85 | 27.1 |
| One specific | 156 | 49.7 |
| Two specific | 62 | 19.7 |
| Three specific | 5 | 1.6 |
| Four specific | 6 | 1.9 |
| Total | 314 | 100.0 |
This study demonstrated a seroprevalence of 13.8% (95% CI 5.9–21.7) of human cysticercosis in the municipality of Jataí, in the southwest of Goiás state. This prevalence characterizes the study area as being endemic for cysticercosis according to the Pan American Health Organization.11 There was a higher seroprevalence among females aged 41–50, but it is known that the parasite's predilection is not correlated with sex per se, but often with food habit or the region of study, among other factors.4,5 The NCC-related mortality rates in Brazil (2000–2011) showed 1.59 deaths/1,000,000 inhabitants for Goiás (Central-West region).5 According to the Brazilian Institute of Geography and Statistics (IBGE), in 2008, in the state of Goiás, only 33% of the households were served by a general sewage system.9 In Goiânia, out of 92 swine blood samples submitted to the indirect ELISA technique, 7.6% were positive for cysticercosis.12 In the municipality of Jataí it is easy to find households where pigs are raised in the peridomicile or on farms, which is associated with other risk factors, such as precarious sanitary and low socioeconomic conditions, which may explain the high seroprevalence of cysticercosis. Our seroprevalence of cysticercosis is higher than the 11.3% found in another study in the municipality of Catalão, Goiás including 354 serum samples from the population analyzed by ELISA and WB; the serology reactivity was associated with areas without a sewage collection network.13
Cysticercosis is endemic in Brazil according to several studies.4,5 In the northeastern region, a cohort study carried out in the municipality of João da Costa, in Piauí state, showed a prevalence of 13.6% for cysticercosis by ELISA in the first stage of the study in 169 individuals with a confirmed or suspected history of cysticercosis infection. Subsequent analysis of these 92 reagent samples in the first stage revealed that 24% presented reagent samples in the ELISA test and 29% in WB.14 In the city of Mulungu do Morro, in the state of Bahia, the prevalence of human cysticercosis was 1.6% in 694 blood samples collected from the general population; the area is considered an area of casual incidence, although it has characteristics that indicate poor health and socioeconomic profiles.15 In the Southeast region, specific IgG antibodies against cysticercosis were detected in 13.5%, 5.0%, 4.8%, and 4.7% of serum samples from blood donors in the cities of Araguari, Tupaciguara, Monte Alegre de Minas and Uberlândia, respectively, in Minas Gerais state.16 In a study carried out in 1863 inhabitants of the rural area of the municipality of Cássia dos Coqueiros, in São Paulo state, using specific ELISA and WB tests and considering the use of WB as confirmatory due to its high specificity, the anti-cysticercus serum prevalence in this population was 2.1%.17 In another study, conducted in a rural settlement in São Paulo, 5.7% of IgG antibodies were found in 194 serum samples by ELISA test and WB confirmed by 18kDa and 14kDa proteins purified from vesicular fluid metacestodes of T. solium.18 In the South region, a study demonstrated that cysticercosis seroprevalence was 3.4% in Lages city, Santa Catarina state, in which 850 blood samples were collected on filter paper using an antigenic extract of vesicular fluid for testing by WB.19
The high frequency of infection by other parasites in the Brazilian population may interfere with the performance of the ELISA test where a total parasite extract is used, as in the present study, whose results showed a positivity of 66.3%; the total antigenic extract from cysticercus of T. solium correlates with a high cross-reactivity tendency in serum samples from individuals with taeniasis in the ELISA test.10 The use of WB as a confirmatory test for the immunological diagnosis of cysticercosis is justified because of the high specificity of the peptides recognized by IgG antibodies when using total saline extract of T. solium metacestodes.10,13,18 Elevated levels of IgG predominate in serum samples from NCC patients at different evolutionary stages of the disease and constitute an immunological marker of chronic exposure.2
The detection of IgG antibodies remains a reliable method in cysticercosis seroepidemiological studies as it is noninvasive and demonstrates previous exposure of individuals to these parasites. The results suggest that the municipality of Jataí is endemic for cysticercosis and there is a need for health managers to adopt measures to control and prevent this parasitosis.
FundingFundação de Amparo à Pesquisa do Estado de Goiás (FAPEG).
Conflicts of interestThe authors declare no conflicts of interest.
We give our deepest thanks to the participants of the study.