A cross-sectional study was carried out in the Hematology and Hemotherapy Institute of the state of Mato Grosso do Sul (Hemosul) to evaluate the seroprevalence and risk factors of Hepatitis E Virus (HEV) exposure among volunteer blood donors in Central Brazil. Two-hundred fifty samples from the biorepository were tested for anti-HEV IgG and IgM using the Wantai HEV ELISA test. The seroprevalence of HEV exposure was 6.4% (95% CI: 3.9–10.2). Being born in another state of Brazil, mainly in the Southeast and South regions, was associated with a higher risk of HEV exposure (p < 0.001).
Dear editor
Hepatitis E Virus (HEV) infection is an important cause of enteric acute viral hepatitis worldwide, being a serious global public health problem in many countries.1 This infection is mostly asymptomatic and self-limiting; however fulminant hepatic failure can occur in women with acute HEV infection during pregnancy. HEV infection can also cause chronic hepatitis and extrahepatic illnesses in immunocompromised individuals.2,3
HEV infection spreads through the fecal-oral route and is transmitted primarily via the consumption of contaminated food, water, or uncooked/undercooked meat.4 The prevalence of HEV exposure differs between and within developing and developed countries, and is determined by geographical area, eating and hygiene habits, and environmental factors. The World Health Organization (WHO) estimates that every year 20 million people are infected with HEV worldwide. More than 3.3 million symptomatic cases of acute HEV infection, causing 70,000 deaths, are reported annually.5
A recent meta-analysis reported a seroprevalence of exposure to HEV of 6.0% in the Brazilian adult population, but the seroprevalence in blood donors varies from 0.4% to 18.7%.6,7 In addition, discrepancies in the sensitivity and specificity of diagnostic tests may have underestimated the HEV exposure prevalence and biased epidemiological data and prevention measures.8
Transfusion-transmitted HEV infections represent a risk for immunosuppressed patients and could be a cause of severe or fatal complications.9 As routine testing of blood products for HEV infection has not been implemented in Brazil and data regarding the epidemiology of HEV exposure in Central Brazil are limited or outdated, this study aimed to evaluate the seroprevalence of HEV exposure among volunteer blood donors from the state of Mato Grosso do Sul in Central Brazil.
This cross-sectional survey included information from databases and 250 samples from the biorepository of a previous research project10 with sufficient volume to perform the tests. The participants were volunteer blood donors consecutively recruited during the period from April to November 2011 at Hemotherapy Institute “José Scaff” – Hemosul in Campo Grande, the capital city of Mato Grosso do Sul state, Brazil. Participants were first informed about the project and asked to sign an informed consent form prior to being interviewed using a standardized questionnaire. All serum samples were tested by a commercially available ELISA immunoassay (Wantai Beijing, China) for the presence of anti-HEV IgG and IgM. Evidence of HEV exposure was defined as a positive anti-HEV IgG and/or IgM test result.
The prevalence of HEV exposure was calculated with a 95% Confidence Interval (95% CI). Chi-Square, Fisher's exact test, and logistic regression were used to determine the association between HEV exposure and each variable/risk factor estimating the Odds Ratios (ORs). The variables that presented a p-value < 0.20 were included in multiple logistic regression models, according to the number of Events Per Variable (EPV). A p-value < 0.05 were considered significant. All analyses were performed using the STATA V.13.0 software (Stata Corp LP, College Station, USA).
This study was carried out following the recommendations of the Ethical Committee on Human Research of the Federal University of Mato Grosso do Sul (protocol number 4.410.223, CAAE 28377719.3.0000.0021).
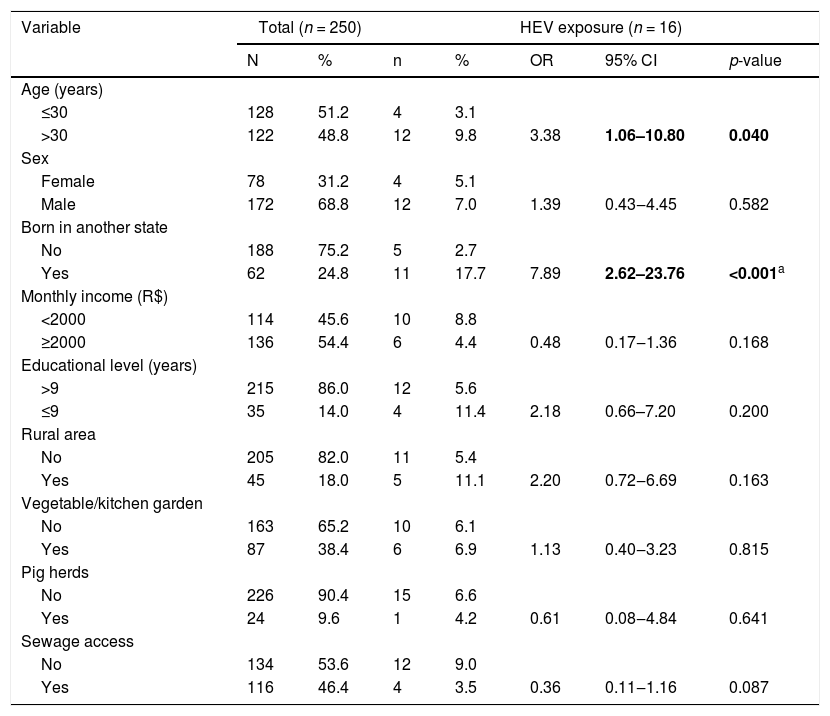
The individuals included in this study were 18‒68 years old (median age: 30 years old) and 68.8% (n = 172) were male. Most of the participants were born in Mato Grosso do Sul state 75.2% (n = 188), 54.4% (n = 136) reported a monthly income ≥ 1,081 USD (median 1,081) and 86% (n = 215) had > 9 years of schooling. In addition, 18.0% (n = 45) reported living in rural area, 38.4% (n = 87) have vegetable/kitchen garden, 9.6% (n = 24) living close to pig herds, 44.8% (n = 112) had contact with wild animals, and 53.6% (n = 134) have no sewage access.
In this study, the overall prevalence of HEV exposure was 6.4% (95% CI: 3.9–10.2). Among 250 samples tested, 16 were anti-HEV IgG positive, and none were anti-HEV IgM positive. The seroprevalence of HEV exposure was higher among blood donors older than 30 years of age (3.1% vs. 9.8%; p < 0.040) and were born in a state other than Mato Grosso do Sul (2.7% vs. 17.7%; p < 0.001). After multiple logistic regression, being born in another state of Brazil remained independently associated with HEV exposure (OR = 6.57; p < 0.001). No association (p > 0.05) was found between the presence of HEV exposure and other sociodemographic variables including sex, educational level, area of residence (rural or urban), income, vegetable/kitchen garden and pig herds, and sewage access (Table 1).
HEV exposure among volunteer blood donors in Mato Grosso do Sul, Central Brazil, 2011.
| Variable | Total (n = 250) | HEV exposure (n = 16) | |||||
|---|---|---|---|---|---|---|---|
| N | % | n | % | OR | 95% CI | p-value | |
| Age (years) | |||||||
| ≤30 | 128 | 51.2 | 4 | 3.1 | |||
| >30 | 122 | 48.8 | 12 | 9.8 | 3.38 | 1.06–10.80 | 0.040 |
| Sex | |||||||
| Female | 78 | 31.2 | 4 | 5.1 | |||
| Male | 172 | 68.8 | 12 | 7.0 | 1.39 | 0.43‒4.45 | 0.582 |
| Born in another state | |||||||
| No | 188 | 75.2 | 5 | 2.7 | |||
| Yes | 62 | 24.8 | 11 | 17.7 | 7.89 | 2.62–23.76 | <0.001a |
| Monthly income (R$) | |||||||
| <2000 | 114 | 45.6 | 10 | 8.8 | |||
| ≥2000 | 136 | 54.4 | 6 | 4.4 | 0.48 | 0.17‒1.36 | 0.168 |
| Educational level (years) | |||||||
| >9 | 215 | 86.0 | 12 | 5.6 | |||
| ≤9 | 35 | 14.0 | 4 | 11.4 | 2.18 | 0.66–7.20 | 0.200 |
| Rural area | |||||||
| No | 205 | 82.0 | 11 | 5.4 | |||
| Yes | 45 | 18.0 | 5 | 11.1 | 2.20 | 0.72‒6.69 | 0.163 |
| Vegetable/kitchen garden | |||||||
| No | 163 | 65.2 | 10 | 6.1 | |||
| Yes | 87 | 38.4 | 6 | 6.9 | 1.13 | 0.40‒3.23 | 0.815 |
| Pig herds | |||||||
| No | 226 | 90.4 | 15 | 6.6 | |||
| Yes | 24 | 9.6 | 1 | 4.2 | 0.61 | 0.08‒4.84 | 0.641 |
| Sewage access | |||||||
| No | 134 | 53.6 | 12 | 9.0 | |||
| Yes | 116 | 46.4 | 4 | 3.5 | 0.36 | 0.11‒1.16 | 0.087 |
To our knowledge, this is the first HEV epidemiological study including blood donors in Central Brazil. The prevalence we found is lower than the only study previously reported from Mato Grosso do Sul, which evaluated the HEV prevalence among people who used crack cocaine 14.2% (95% CI 11.8%–17.0%).11 Both studies used the Wantai HEV ELISA kits to perform the tests, and this difference could be explained by the risk behaviors of people who use crack cocaine such as shared use of crack-cocaine equipment, socioeconomic status, environmental sanitation, and poor personal hygiene, which facilitates the transmission of diseases via the fecal-oral route.11,12
A recent meta-analysis demonstrated an estimated overall HEV exposure prevalence of 6.0% among adults in Brazil, which is similar to the seroprevalence found in this study (6.4%).7 However, screening studies carried out among Brazilian blood donors show large differences with seroprevalence ranging from 0.4% to 18.7%.6,7 Differences in the sensitivity and specificity of the anti-HEV assays used, sample size, population, and region, could explain the discrepancies in the rates of HEV exposure published in the various previous studies in Brazil. It is worth mentioning that the kit used in this study has been reported to have high sensitivity (>99%) and specificity (>95%),8 improving the prevalence data.13
In this study the HEV exposure was associated with blood donors who were born in a state other than Mato Grosso do Sul (p = 0.001). Among 16 anti-HEV positive participants, 9 (56.2%) were from the Southeast or South regions of Brazil, where several studies have reported the presence of HEV RNA in swine herds and high prevalence of HEV exposure among blood donors and general population. The cultural high consumption of raw and undercooked pork meat, especially in the South region, is already known to be the major transmission route for HEV infection in Brazil.14 A previous study in the South region including blood donors performed with the same commercial assay reported a HEV prevalence of 18.7%,6 and another study reported a prevalence greater than 55% using an indirect in-house ELISA assay.15 Therefore, consumption of raw or undercooked pork meat seems to be the main route of HEV transmission in Brazil and our results support this evidence as most of the anti-HEV positive participants declared being born in regions with high consumption of pork meat.
This study has some limitations, including a small sample size and participants' recall bias when answering the questionnaires. As the analyzed specimens were from a biorepository collected in 2011, new studies are needed to verify the current HEV prevalence among general population in the state of Mato Grosso do Sul. In addition, information related to drinking-water, ingestion of raw and undercooked meat such as wild boar, deer, or pork were not assessed. Despite these limitations, this study is the first research on the seroprevalence of HEV exposure among blood donors in Central Brazil, enriching the knowledge of HEV infection in the country.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
The institutions at which the work was performed: UFMS and FIOCRUZ-MS, Campo Grande: conceive and design the study, collect, and store the blood samples, statistical analysis and draft the article. UNIFESP: serological analysis.