Rapid Diagnostic Tests (RDT) are useful to identify syphilis cases, particularly for hard-to-reach populations and if laboratory services are scarce. However, RDT performance may be suboptimal. We aimed to assess the sensitivity and specificity of a syphilis RDT using well-characterized blood donors’ samples. We categorized samples from 811 blood donors into five groups: 1 - Samples with reactive Chemiluminescence (QML), FTA-Abs, and VDRL; 2 - Samples with reactive QML and FTA-Abs, and nonreactive VDRL; 3 - Samples with reactive QML, and nonreactive for other markers (false-positives); 4 - Controls with nonreactive QML; and 5 - Samples reactive for HIV, with nonreactive QML. Sensitivity was tested in groups 1 (overall and according to VDRL titers) and 2; specificity was tested in groups 3‒5. The RDT had high specificity, even in samples reactive for HIV. The sensitivity was high (91.9%) in samples with reactive VDRL but varied between 75.0%‒100% according to VDRL titers. The overall sensitivity was lower (81.3%) in samples with reactive FTA-Abs and nonreactive VDRL. The RDT is a useful tool to detect active syphilis but may be more limited for cases with very early or remote infection, or those with prior treatment. When higher sensitivity is needed, additional strategies including recurrent testing or laboratory-based tests may be required.
Syphilis is a sexually transmitted, bacterial infection that persists as a significant public health problem despite the availability of an effective and affordable treatment for more than 70-years.1 The incidence of syphilis has been increasing in several settings, particularly in low and middle-income countries and among vulnerable populations, including people living with HIV.2,3 In addition to the morbidity associated with clinical manifestations in both sexually-acquired and congenital infections, untreated syphilis can significantly increase the risk of HIV acquisition; furthermore, syphilis-HIV coinfection has been associated with more aggressive manifestations than either infection alone.4
Clinical manifestations of syphilis are often uncharacteristic or may be completely absent, highlighting the crucial importance of diagnostic tests to identify and treat individuals who inadvertently participate in transmission chains. In clinical settings, particularly when dealing with hard-to-reach populations and scarce laboratory services, point-of-care tests are invaluable tools allowing immediate diagnosis and treatment. The adoption of point-of-care, Rapid Diagnostic Tests (RDT) for several health conditions is in rapid progress, underscoring the need for reliability and accuracy assessments of different tests using real-life samples.5
In a recently published systematic review, Zhang et al. evaluated 19 studies addressing the performance of RDT for syphilis, including a meta-analysis of 13 studies. Although the pooled sensitivity and specificity of the treponemal component were high (93% and 98% respectively), there was a high variability across studies, from 48% to 100% in sensitivity, and 62% to 100% in specificity. In addition, results were not stratified by syphilis stages, which likely influence the overall performance of RDT.6 Few studies have addressed the accuracy of syphilis RDT among people living with HIV;7 this is a relevant issue since previous studies suggest higher risk of false-positive syphilis reactivity in this population using different diagnostic tools.8
In this study, we aimed to assess the sensitivity and specificity of a treponemal RDT routinely used in primary care settings in Brazil for syphilis diagnosis using samples from blood donors previously tested with traditional laboratory-based treponemal and non-treponemal serologic assays. We also explored the performance of the RDT in samples with positive test results for HIV, to address potential variations in RDT specificity in this subgroup.
The blood bank at (anonymized information) in São Paulo, Brazil, routinely selects potential blood donors with an interview addressing exposure to transfusion-transmissible infections followed by a comprehensive laboratory screening. We identified stored blood samples from volunteer, healthy blood donors aged 18-years old and older who underwent the screening interview between 2017‒2021 and had available information on demographics and laboratory test results for syphilis and HIV. Serologic tests for HIV and syphilis Chemiluminescence (QML) were performed using the ARCHITECT i2000SR immunoassay analyzer (ABBOTT Diagnostics, Chicago, USA). HIV was also tested using Real-Time Polymerase Chain Reaction (RT-PCR) tests from COBAS® S201 System, Roche NAT instruments (Roche, Basel, Switzerland) according to the manufacturer's instructions. Samples with reactive results in the initial treponemal syphilis screening with QML underwent confirmatory testing for treponemal reactivity using FTA-Abs indirect immunofluorescence (Wama diagnostica, São Carlos, Brazil); samples were subsequently tested with the venereal disease research laboratory (VDRL) flocculation assay to assess non-treponemal reactivity (Wama diagnostica, Sao Carlos, Brazil). We categorized the samples included in this study into 5 groups: 1 - Donors with reactive results in QML, FTA-Abs, and VDRL; 2 - Donors with reactive QML and FTA-Abs, with negative VDRL; 3 - Donors with reactive QML, and negative results for other markers (false positives); 4 - Controls with nonreactive QML; and 5 - Donors with positive test results for HIV and nonreactive QML. FTA-Abs were considered the reference (gold-standard) treponemal test. All samples were thawed, centrifuged, and submitted to the syphilis RDT (RT Syphilis Bio, Bioclin, Belo Horizonte, Brazil), an immunochromatographic treponemal assay for qualitative detection of IgG and IgM, according to the manufacturer's instructions.
Descriptive analysis was performed, including frequencies and percentages for categorical variables, as well as medians and interquartile ranges for numeric variables. Specificity was calculated separately for samples from groups 4 and 5, while sensitivity was calculated separately for groups 1 and 2. We also estimated RDT positivity and specificity in group 3, and sensitivity estimates in group 1 based on VDRL titers when available. For each indicator, we present precision estimates using 95% Confidence Intervals (95% CI). We identified and included in the study all available samples for groups 1, 2, 3, and 5 to obtain the highest possible precision in estimates; additionally, we planned to include at least 300 samples in group 4 to obtain specificity estimates with a total width of 95% CI of 1.2%.
The institutional Ethics Committee revised and approved this study with exemption of informed consent (approval number 4.528.454). All participants’ identifiable information was kept confidential throughout the study.
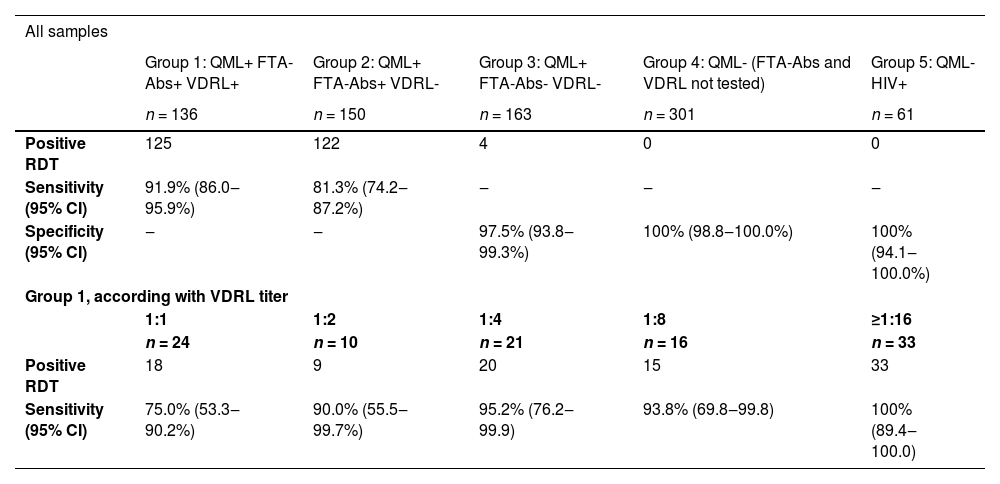
A total of 811 samples were included in the study; groups 1‒5 included, respectively, 136, 150, 163, 301, and 61 participants. Most donors (58%) were males, with a median age of 36-years old (interquartile range 28‒46), and 64% declared Caucasian race/ethnicity. Table 1 presents estimates of sensitivity and specificity for each group, as well as estimates according to VDRL titers in group 1. The overall sensitivity of the RDT was 91.9% in group 1, but only 81.3% in group 2. In group 3, 4 of 163 samples (2.4%) were reactive in the RDT, representing a 97.5% specificity in this group. Both groups 4 and 5 had 100% specificity. In the analysis according to the VDRL titer in group 1, we found that sensitivity varied between 75.0% among donors with a 1:1 titer, and 100% among donors with titers ≥1:16.
Sensitivity and specificity of rapid diagnostic test for syphilis according to groups defined by serologic profile.
Group 3: positivity 4/163 (2.4%; 95% CI 0.7‒6.2%).
QML, Chemiluminescence.
In this study, we found that the RDT had a very high specificity, with only 2.4% false-positive results in samples with a positive QML and negative FTA-Abs/VDRL, and no false positive results in samples with a negative QML. The overall sensitivity of the RDT was high (91.9%) in blood donor samples with reactive VDRL but varied between 75.0% and 100% according to VDRL titers. The overall sensitivity was lower (81.3%) in samples with a positive FTA-Abs and negative VDRL. These findings suggest that the RDT is a useful tool to detect active cases of syphilis but may have a more limited applicability for cases with very early or remote incident infection, or those with prior treatment. Finally, we found that the specificity of the RDT was very high in samples with positive test results for HIV.
Our findings support results from studies addressing other syphilis RDT that showed increased sensitivity when including a reactive non-treponemal test as part of the reference diagnostic workup.9 They are also reassuring for the specificity of this RDT in people living with HIV.
Our study had a few limitations regarding the specimens and the population included for analysis. We used thawed serum samples, which differ from fresh whole blood samples that are usually employed in point-of-care RDT. Since comparative studies suggest that RDT sensitivity in whole blood specimens may be lower when compared to serum samples,10 the test sensitivity observed in our study may be an overestimation of true values. As for the population included in the study, blood donors are often considered healthier than the general population; it is plausible to assume that populations with higher prevalence of comorbidities and coinfections could have a higher prevalence of false positive results due to the presence of cross-reactive antibodies. Finally, we were unable to include samples from donors with reactive results for both HIV and syphilis in our sensitivity analysis.
Despite these limitations, our study included a large number of well-characterized samples and demonstrated a consistent trend of lower sensitivity of the RDT in samples with lower or negative VDRL titers. Although our findings suggest that the RDT is a robust tool to detect active syphilis cases, certain conditions that require higher test sensitivity, such as antenatal care, may benefit from strategies such as recurrent testing and concurrent use of conventional laboratory-based tests. Our results also provide useful information for the development of testing policies in Brazil as well as other countries. The implementation of robust point-of-care tests is a key strategy to control the dissemination and detrimental outcomes associated with syphilis.
Data sharingCurrently, the datasets used and/or analysed during the study are available from the corresponding author on reasonable request.
Ethics approvalThe institutional Ethics Committee revised and approved this study with exemption of informed consent (approval number 4.528.454). All participants’ identifiable information was kept confidential throughout the study.
Authors’ contributionsCSA, JALM, MVA and VAS conceived the study. CBB and LDS identified samples to be included in the analysis. CSA and JALM performed the syphilis rapid tests. VAS performed data analysis. MVA, AEM, LDS, CBB and VAS contributed with interpretation of results. CSA, JALM, MVA and VAS wrote the first draft of the manuscript. All authors revised and approved the final version of the manuscript.
FundingThis research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
We thank the Ministry of Health of Brazil for the donation of RDT kits for this study.