Over-the-counter use of ivermectin amongst other drugs as SARS-CoV-2 treatment has been increasingly common, despite the lack of evidence on its clinical efficacy.
ObjectiveTo evaluate the effect of ivermectin use on production of antibodies against SARS-CoV-2 in health care workers (HCW) diagnosed with COVID-19 and of Th1/Th2 cytokines by stimulated peripheral blood mononuclear cells of the same cohort (PBMCs).
MethodsThis cross-sectional study evaluated seroconversion and neutralizing antibodies production in HCW at Complexo Hospitalar Universitário Professor Edgard Santos (Salvador, Brazil), diagnosed with COVID-19 from May to July, 2020, as well as in vitro production of antibody against SARS-CoV-2 and Th1/Th2 cytokines. Analyses were performed between December 2020 and February 2021. Participants were stratified according to the use of ivermectin (≤ 1 dose vs. multiple doses) for treatment of COVID-19.
Results45 HCW were included (62% women). Mean age was 39 years, and disease severity was similar across groups. Neutralizing antibodies were detected less frequently in multiple doses (70%) vs. ≤ 1 dose (97%) groups, p = 0.02). PBMCs of patients in multiple doses group also were less likely to produce antibodies against SARS-CoV-2 following in vitro stimulation with purified spike protein in comparison with patients in ≤ 1 dose group (p < 0.001). PBMC´s production of Th1/Th2 cytokines levels was similar across groups. Abdominal pain (15% vs 46%, p = 0.04), diarrhea (21% vs. 55%, p = 0.05) and taste perversion (0% vs. 18%, p = 0.05) were more frequently reported by participants that used multiple doses of ivermectin.
ConclusionsAlthough there was no evidence for differential disease severity upon ivermectin use for treatment of COVID-19 it was associated with more gastro-intestinal side-effects and impairment of anti-SARS-CoV2 antibodies production, in a dose dependent manner. This potentially impacts the effectiveness of immune response and the risk of reinfection and warrants additional studies for clarifying the mechanisms and consequences of such immunomodulatory effects.
Ivermectin is a medication of the avermectin family of compounds that is FDA-approved to treat parasite infections.1 A preliminary in vitro study on the effect of ivermectin against SARS-CoV-2,2 caused a huge increase in its over-the-counter use, despite the lack of evidence of clinical efficacy. Ivermectin has a controversial immunomodulatory effect in animal models, either increasing antibody production and white-blood-cell count or decreasing antibody levels in a dose dependent response.2,3
Seroconversion rates and durability of specific antibodies in SARS-CoV-2 infection are not completely understood. Impaired production of antibodies against COVID-19 can affect disease severity, and likelihood of reinfection.4–6 Understanding the immune response in COVID-19 patients is essential for its treatment and prevention.
This study investigates the effects of self-prescribed use of different ivermectin doses on rates of seroconversion for SARS-CoV-2 in health care workers (HCW) diagnosed with COVID-19, and the ability of HCW´ peripheral blood mononuclear cells (PBMCs) to present an in vitro-induced IgG antibodies (IVIAP) positive test, as well as to produce Th1/Th2 cytokines.
MethodsIn this cross-sectional study we included HCW diagnosed with COVID-19 from May to July 2020 at Professor Edgard Santos University Hospital, in Salvador-Brazil. SARS-CoV-2 infection was confirmed by RT-PCR test (Charité-Berlin protocol).7 This study was approved by the Institutional Research Ethics Committee (No. 4.042.620).
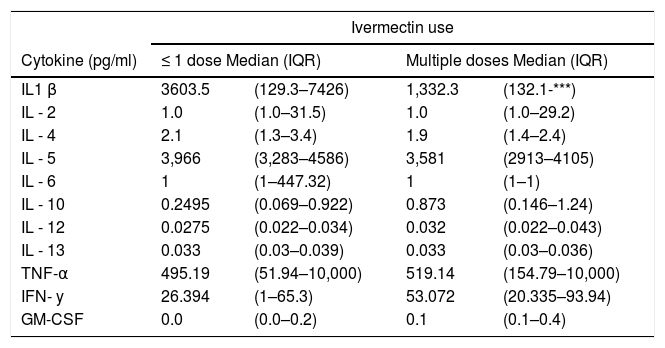
After signing the informed consent form, participants donated 10 ml of blood to perform laboratory tests and filled out a questionnaire on demographics, clinical characteristics, medications and ivermectin dosages used during disease. We stratified HCW by use of ivermectin (no use/single dose vs multiple doses). Each dose corresponds to one 6 mg pill of ivermectin.
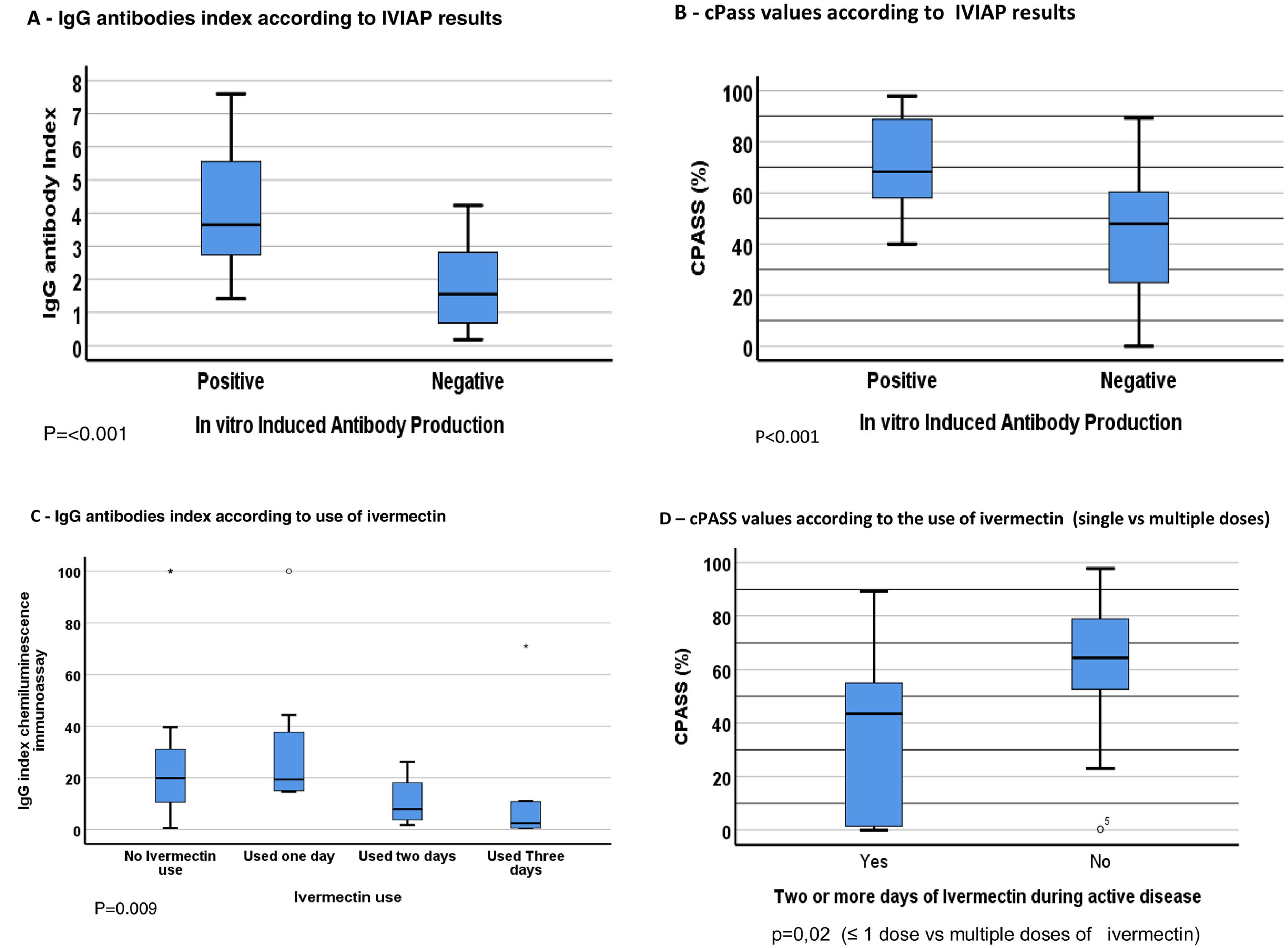
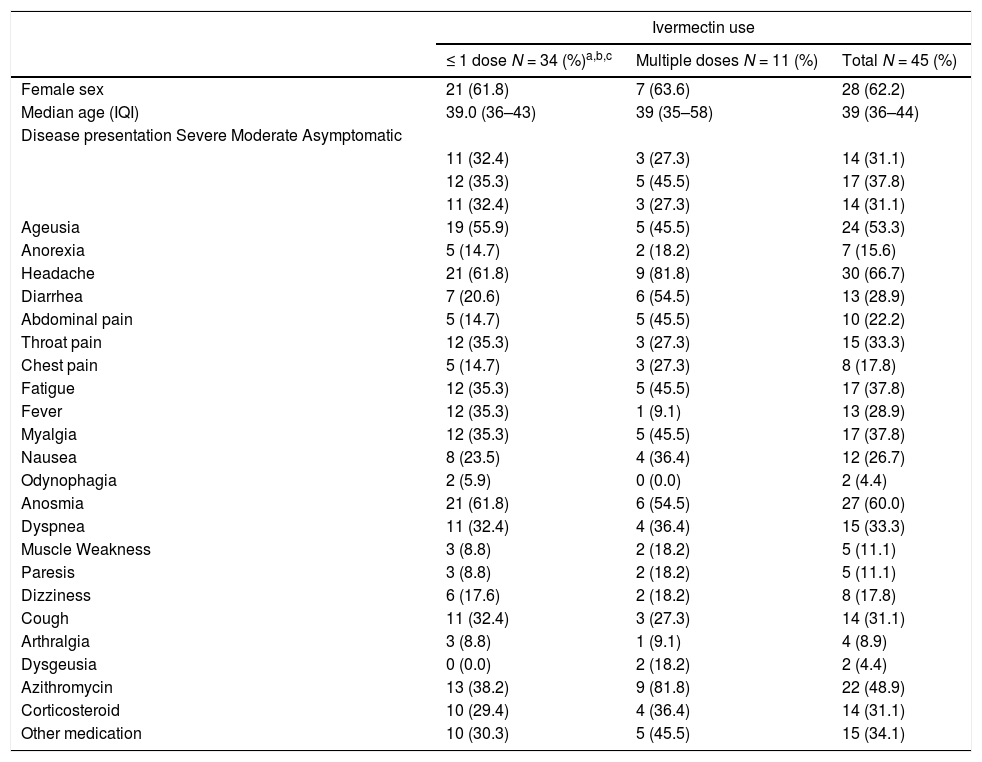
Samples were screened using a commercially available in vitro immunoassay (2019-nCoV IgG kit, SNIBE diagnostics, Shenzhen, China) to detect SARS-CoV-2-specific immunoglobulins against full-length spike and nucleocapsid proteins. A result greater than or equal to 1.00 AU/mL was considered reactive. Next, a cPass Neutralization Antibody Detection kit (Genscript, Leiden, the Netherlands) was used to detect circulating neutralizing antibodies against SARS-CoV-2 that block the interaction between the receptor binding domain (RBD) of the viral spike glycoprotein with the ACE2 cell surface receptor. A ratio greater than 20% was considered positive, according to manufacturer´s instructions.
An in vitro Induced Antibody Production (IVIAP) test was used to identify the ability of HCW´s PBMC in producing IgG specific antibodies against SARS-CoV-2, and 11 different cytokines, after stimulation with the peak spike protein.8,9 PBMCs were separated by a Ficoll-Hypaque gradient, washed three times and adjusted to 2 × 105 cells/100 uL/well in RPMI 1640 medium containing 10% fetal bovine serum. PBMCs were cultured in an Anti-SARS-CoV-2 IgG ELISA microplate for 24 hours at 37°C and 5% CO2. After 24 h, levels of IL1 beta, IL2, IL4, IL5, IL6, IL10, IL12, P70IL13, TNF-alpha, IFN-y and GM-CSF were measured in culture´s supernatant (Human Th1/Th2 Magnetic Luminex Performance Assay 11-plex Fixed Panel Summary, R&D Systems, Minneapolis, MN, USA). Detection of IgG antibodies against SARS-CoV-2 was performed in the microplates used to culture the PBMCs. Mean elapsed time between diagnosis of COVID-19 and study´s tests was 109 days, and was similar for both groups.
To calculate the sample size we estimated that 75% of COVID-19 patients would have a positive IVIAP test, and only 30% of those using ivermectin would do so. A total of 46 participants would provide 80% power, 95% confidence intervals (CI) for the study. Mann-Whitney rank test was used to compare medians of IgG antibody Index and cPASS versus IVIAP (positive/negative) and use of Ivermectin (1 ≤ dose versus multiple doses). Fisher exact text (bicaudal) was used to compare ivermectin use versus ELISA, IVIAP, cPASS (less than 20%, as negative, 20% or more, positive) results.
ResultsA total of 45 HCW were evaluated. Mean time between COVID-19 diagnosis and laboratory testing was similar for IVIAP positive (110.7±31.8 days) or negative HCW (112 ± 65.5 days; p = 0.3). The most used medications during acute disease were ivermectin, azithromycin, and oral corticosteroids. Ivermectin was used by 19 HCW (8 used a single dose, 11 used multiple doses). There was no significant difference in disease severity for HCW who used ivermectin compared to those who did not. However, abdominal pain, diarrhea, and taste perversion were more frequent in HCW that used multiple doses of ivermectin (Table 1).
Clinical and demographic characteristics of health care workers diagnosed with COVID-19, by ivermectin use.
| Ivermectin use | |||
|---|---|---|---|
| ≤ 1 dose N = 34 (%)a,b,c | Multiple doses N = 11 (%) | Total N = 45 (%) | |
| Female sex | 21 (61.8) | 7 (63.6) | 28 (62.2) |
| Median age (IQI) | 39.0 (36–43) | 39 (35–58) | 39 (36–44) |
| Disease presentation Severe Moderate Asymptomatic | |||
| 11 (32.4) | 3 (27.3) | 14 (31.1) | |
| 12 (35.3) | 5 (45.5) | 17 (37.8) | |
| 11 (32.4) | 3 (27.3) | 14 (31.1) | |
| Ageusia | 19 (55.9) | 5 (45.5) | 24 (53.3) |
| Anorexia | 5 (14.7) | 2 (18.2) | 7 (15.6) |
| Headache | 21 (61.8) | 9 (81.8) | 30 (66.7) |
| Diarrhea | 7 (20.6) | 6 (54.5) | 13 (28.9) |
| Abdominal pain | 5 (14.7) | 5 (45.5) | 10 (22.2) |
| Throat pain | 12 (35.3) | 3 (27.3) | 15 (33.3) |
| Chest pain | 5 (14.7) | 3 (27.3) | 8 (17.8) |
| Fatigue | 12 (35.3) | 5 (45.5) | 17 (37.8) |
| Fever | 12 (35.3) | 1 (9.1) | 13 (28.9) |
| Myalgia | 12 (35.3) | 5 (45.5) | 17 (37.8) |
| Nausea | 8 (23.5) | 4 (36.4) | 12 (26.7) |
| Odynophagia | 2 (5.9) | 0 (0.0) | 2 (4.4) |
| Anosmia | 21 (61.8) | 6 (54.5) | 27 (60.0) |
| Dyspnea | 11 (32.4) | 4 (36.4) | 15 (33.3) |
| Muscle Weakness | 3 (8.8) | 2 (18.2) | 5 (11.1) |
| Paresis | 3 (8.8) | 2 (18.2) | 5 (11.1) |
| Dizziness | 6 (17.6) | 2 (18.2) | 8 (17.8) |
| Cough | 11 (32.4) | 3 (27.3) | 14 (31.1) |
| Arthralgia | 3 (8.8) | 1 (9.1) | 4 (8.9) |
| Dysgeusia | 0 (0.0) | 2 (18.2) | 2 (4.4) |
| Azithromycin | 13 (38.2) | 9 (81.8) | 22 (48.9) |
| Corticosteroid | 10 (29.4) | 4 (36.4) | 14 (31.1) |
| Other medication | 10 (30.3) | 5 (45.5) | 15 (34.1) |
In vitro cytokines production according to the ivermectin dose used by patients.
| Ivermectin use | ||||
|---|---|---|---|---|
| Cytokine (pg/ml) | ≤ 1 dose Median (IQR) | Multiple doses Median (IQR) | ||
| IL1 β | 3603.5 | (129.3–7426) | 1,332.3 | (132.1-***) |
| IL - 2 | 1.0 | (1.0–31.5) | 1.0 | (1.0–29.2) |
| IL - 4 | 2.1 | (1.3–3.4) | 1.9 | (1.4–2.4) |
| IL - 5 | 3,966 | (3,283–4586) | 3,581 | (2913–4105) |
| IL - 6 | 1 | (1–447.32) | 1 | (1–1) |
| IL - 10 | 0.2495 | (0.069–0.922) | 0.873 | (0.146–1.24) |
| IL - 12 | 0.0275 | (0.022–0.034) | 0.032 | (0.022–0.043) |
| IL - 13 | 0.033 | (0.03–0.039) | 0.033 | (0.03–0.036) |
| TNF-α | 495.19 | (51.94–10,000) | 519.14 | (154.79–10,000) |
| IFN- y | 26.394 | (1–65.3) | 53.072 | (20.335–93.94) |
| GM-CSF | 0.0 | (0.0–0.2) | 0.1 | (0.1–0.4) |
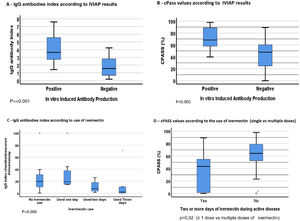
Seroconversion for SARS-CoV-2 was detected in 42/45 (93%) HCW. SARS-CoV-2 antibodies were detected in 8/10 (80%) HCW that used multiple doses of ivermectin, versus 33/34 (97%) of those that used ≤ 1 dose (p = 0.1; One patient in multiple doses group was not tested because of insufficient sample). IVIAP test was positive in 24/45 (53%) HCW, all of them in ≤ 1 dose group (p < 0.001). In addition, HCW in multiple dose ivermectin group had significantly lower antibodies levels than those in ≤ 1 dose group (p < 0.009). Neutralizing antibodies against SARS-CoV-2 were detected in 39 (91%) participants (97% in 1 ≤ dose vs. 70% in >1 dose groups; p = 0.03). cPass values were also significantly higher for patients with an IVIAP positive test than for those with a negative result (p < 0.001). Serological results are summarized in Fig. 1. There was no difference in cytokines production across groups. SARS-CoV-2 viral load did not differ among HCW who used ≤ 1 dose (CT: 31.1 ± 4.7) or multiple doses of ivermectin (CT = 29.8 ± 4.7; p = 0.37).
DiscussionWe showed that self-prescribed, multiple doses ivermectin use was significantly associated with lower frequency and levels of neutralizing antibodies against SARS-CoV-2. In addition, PBMCs of most individuals who used multiple doses of ivermectin, failed to produce SARS-CoV-2 IgG antibodies when exposed to the recombinant protein S1 in a dose-dependent fashion.
Ivermectin is a macrocyclic lactone that has direct action on PAK-1, interfering in phosphorylation events, essential for a large number of cellular processes.10 Immunosuppressants derived from macrocyclic lactones (like Everolimus and Sirolimus), act in the Mtor activation domain, resulting in inhibition of antigen-induced T lymphocyte activation and proliferation, as well as cytokine blockage, also inhibiting antibody production. High doses of ivermectin could induce such effects and interfere with antigen recognition and immune memory formation.11,12
Ivermectin was able to inhibit replication of several RNA viruses (including SARS-CoV-2) in in vitro studies.13,14 However, the inhibitory capacity (IC 50) of ivermectin only occurs in extremely high concentrations, 50–100 times that detected in plasma of an adult weighing 70 kg after a single dose of 200 μg/kg.15 Co-administration of ivermectin and azithromycin promotes an increase of 31% in maximum concentration (Cmax) and 27% in the maximum time (Tmax) of stay in the AUC of ivermectin.16 In our study, we found that among 25/45 (55.5%) patients who presented a negative IVIAP test, 14 (56%) of them used ivermectin, 12 (86%) for three or more days, often in association with azithromycin.
Our findings demonstrate that use of multiple doses of ivermectin in COVID-19 patients was significantly associated with lower frequency/lower levels of neutralizing antibodies and reduced production of SARS-CoV-2 antibodies by stimulated PBMCs. No effect was observed on Th1/Th1 cytokines production by stimulated cells, suggesting the effect could be associated with interference on B cells activation or immune memory formation.
The lack of evaluation of specific cellular population involved in immune response to SARS-CoV-2, and the relatively small sample size limit the reach of our conclusions. Nevertheless, our results strongly suggest that ivermectin use can interfere in the specific immune response to SARS-CoV-2 infection, affecting memory cells and the production of antibodies against that virus. Further studies are needed to understand how this effect is promoted by ivermectin use, and what are the consequences for post-infection immunity.
FundingThe study was not associated with any research grant or other funding support.
We would like to express our deepest thanks to the HCW at Professor Edgard Santos University Hospital for their participation, that made this study possible.