Brazil is the third country most affected by Coronavirus Disease 2019 (COVID-19) in the world. Health care workers (HCWs) are at higher risk of infection. Despite the increasing numbers of studies on the topic, There are gaps in the knowledge of characteristics and risk factors for infection of HCWS. This information is important to design preventive strategies and to mitigate the disease impact. The objective of this study was to estimate the incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, to identify factors associated, and to describe symptoms reported by healthcare workers at a tertiary hospital in Salvador, Brazil.
MethodsAll HCWs were evaluated in a cross-sectional study conducted between May and September 2020, using self-administered questionnaires, and screening all participants for SARS-COV-2 IgG and IgM antibodies by rapid tests. Reactive IgG samples were retested by ELISA and IgM-positive test had a saliva sample retest by RT-PCR. Univariate associations were estimated by a non-adjusted incidence proportion ratio. Variables associated with COVID-19 incidence at p < 0.20 were selected for inclusion in a binary logistic regression model.
ResultsA total of 2083 HCWs were included, mean age 41±10 years, 71.8% women, and 77.8% non-white. Of these, 271 (13.0%) and 25 (1.2%) HCWs tested positive for IgG and IgM SARS-CoV-2 antibodies, respectively, and three had a positive RT-PCR. Ancillary work [Odds Ratio (OR): 4.96], elementary education (OR: 2.91), high school education (OR: 2.89), and catholic religion (OR: 2.16) were associated with an increased likelihood of a positive IgG antibodies against SARS-CoV-2. Anosmia [Incidence Proportion Ratio (IPR): 7.41] and ageusia (IPR:8.51) were the most frequent associated symptoms.
ConclusionHCWs with low mean family income, lower level of schooling, ancillary workor being black had a significantly higher likelihood of testing positive for SARS-CoV-2 antibodies. Social vulnerability was an important risk factor for COVID-19 infection.
Coronavirus Disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a new beta-coronavirus that shares 88% of sequence identity from SARS-CoV, the virus that caused an important outbreak in 2002-2003.1-2 COVID-19 is spread worldwide, and Brazil was the country with the third highest number of accumulated cases in the world by the time this study was conducted, according to the Brazilian Ministry of Health´s official report.3
SARS-CoV-2 is transmitted mainly through inhalation or contact with infected droplets. Health care workers (HCWs) have been one of the groups most affected by the pandemic,4,5 due to their position on the front-line, especially those who work in intensive care units where contact with infected SARS-CoV-2 patients is more direct and frequent.6 A similar situation was observed in the 2002 SARS outbreak when 21% of infected patients were HCWs.7 By September 2020, a total of 1405 Brazilian HCWs had been infected by SARS-CoV-2 and 315 died due to COVID-19. The most frequently infected HCWs were nurse technicians (109,955; 34.1%), nurses (47,339; 14.7%), doctors (33,032; 10.3%), community health workers (16,546; 5.1%), and health units´ receptionists (14,024; 4.4%).8 In addition, the population of HCWs also involves general service personnel involved in cleaning, transportation, and food preparation, which are essential workers for the maintenance of hospital´s functioning but generally are not included in the statistics on infected HCWs.9
The high exposure to SARS-CoV-2, and the increased stress levels that characterize the work in pandemic situations have been well-described.5,10,11 Many COVID-19 studies focused on HCWs mental health 11-12 but we still have gaps in the knowledge of specific characteristics of HCWS and the main risk factors related to COVID-19 infection in this occupational group. Identifying the risk factors for COVID-19 acquisition in hospital settings is an important step to design preventive strategies and to mitigate the impact of COVID-19 on HCWs.13 We aimed to estimate the incidence of SARS-CoV-2 infection, to identify factors associated, and to describe symptoms reported by healthcare workers at a tertiary hospital in Salvador, Brazil.
MethodsStudy design and participantsFrom May to September 2020, during the first months of the pandemic, a cross-sectional study of HCWs at a university hospital was conducted. All HCWs over 18 years of age were invited to participate. Participants were actively working at the hospital, asymptomatic at the moment of inclusion. To rule out any possibility of early stage of infection, asymptomatic/oligosymptomatic by COVID-19 infection, we used a questionnaire to assess the presence of any symptom potentially associated with a viral infection. The interview and blood sample collection were performed at the same day. We used rapid tests for detection of IgG and IgM antibodies against SARS-CoV-2. If a HCW tested positive for IgM antibodies, a RT-PCR test was performed to confirm acute infection, and for implementing quarantine. The institutional review board approved this study under CAAE 31748320.3.1001.5543 N° 4.042.620. All participants signed an informed consent form before enrolling for voluntary participation.
Study locationThis University Hospital, located in the city of Salvador, Brazil, is a large public hospital and outpatient teaching unit. During the pandemic, the hospital established a specific ward and an ICU unit for suspected/confirmed cases of SARS-CoV-2, as a measure to protect patients with multiple comorbidities, immunosuppressed, and bone marrow transplant patients treated at the reference complex. A surveillance program was implemented according to current guidelines to diagnosis and treatment of HCWs.14 Serological screening was conducted in all HCWs and SARS-CoV-2 RT-PCR tests were performed in suspected cases, to allow early isolation and treatment. Suspected cases were tested in the same day (by RT-PCR), and results promptly sent to the respective unit, making possible a rapid diagnosis and implementation of preventive measures. Personal protective equipment, social distance and enforced hands hygiene policies were also implemented at the hospital.
Data collection and variablesData collection was carried out using self-administered structured questionnaires, minutes before performing the rapid test for SARS-CoV-2. Independent variables included sex, age, race (white, racially-mixed, black, and other, according to the Brazilian official report on demographics), educational level, family income (Brazilian minimum wage is approximately 217.18 US dollars/month), HCWs function, religion, and signs and symptoms usually associated with the infection like fever, cough, ageusia, anosmia, and others. The main outcome variable was the incidence of COVID-19 from May to September 2020.
Diagnostic testsAfter completing the standardized questionnaire, a blood sample was collected, and plasma was used to perform the rapid and ELISA tests. We used the COVID-19 IgM/IgG Combo ECO Teste – TR. 008 (ECO Diagnóstica LTDA, Nova Lima, MG, Brazil), based on the lateral flow platform for diagnosing SARS-CoV-2 infection. If the rapid test result was positive for IgG antibodies, the same sample was retested by the Euroimmun ELISA Anti-SARS-CoV-2 IgG (Euroimmun AG - Seekamp, 31 - Luebeck - Germany) methodology, specificity of 96.1% (IC: 90.1–98.8) and sensitivity of 89.5% (75.3–96.4).15 All positive samples, in both diagnostic tests (IgG rapid test and ELISA), were considered positive for SARS-CoV-2 infection. HCW presenting IgM reactive samples were asked to provide saliva samples for SARS-CoV-2 RT-PCR (Charité-Berlin protocol).16-17 HCW testing positive for SARS-CoV-2 IgM antibodies were dismissed from hospital duties and kept isolated until RT-PCR results were available.
Health care workers (HCWs)HCWs functions were classified into eight groups: medical doctors, nurses, diagnostic support (laboratory, pharmacy, and bioimage workers), multiprofessional team (psychologist, dentists, speech therapists, social service, physical therapists, occupational therapists, nutritionists), ancillary nurses, administrative (directory, lawyers, administrative, secretaries, archivists), ancillary workers (cooks, cleaning staff, maintenance, porters, security, laundry, drivers) and medical students (interns). During the pandemic period, all students were asked to stay at home from March through September 2020, when last-year medical students resumed their hospital activities. All students were tested before resuming their activities in the hospital, and this group was included in this study for comparative purposes.
Statistical analysisStudy sample size was not calculated since this study was a hospital census. The data were analyzed with the SPSS 18.0 statistical package and OpenEpi. Participants with ≥ 20% missing data were excluded from analysis. Nominal and continuous variables were described in frequency distributions and measures of central tendency, respectively. The main outcome variable was a positive test for COVID-19. Univariate associations were estimated by the incidence proportion ratio (IPR). The first SARS-CoV-2 case in Salvador, Bahia, was diagnosed in March 2020. The study population was 2083 individuals until September 2020. Therefore, all new cases were measured as IPR.18 Variables associated with COVID-19 incidence at p < 0.20 were included in a binary logistic regression model, and Odds Ratios (OR) were calculated with 95% Confidence Intervals (CI). The analysis was completed based on 1887 observations. Variables with p-values less than 0.05 remained in the final model. The omnibus test evaluated the adequacy of the model, and the Hosmer & Lemeshow test indicated the model goodness-of-fit to the data.19
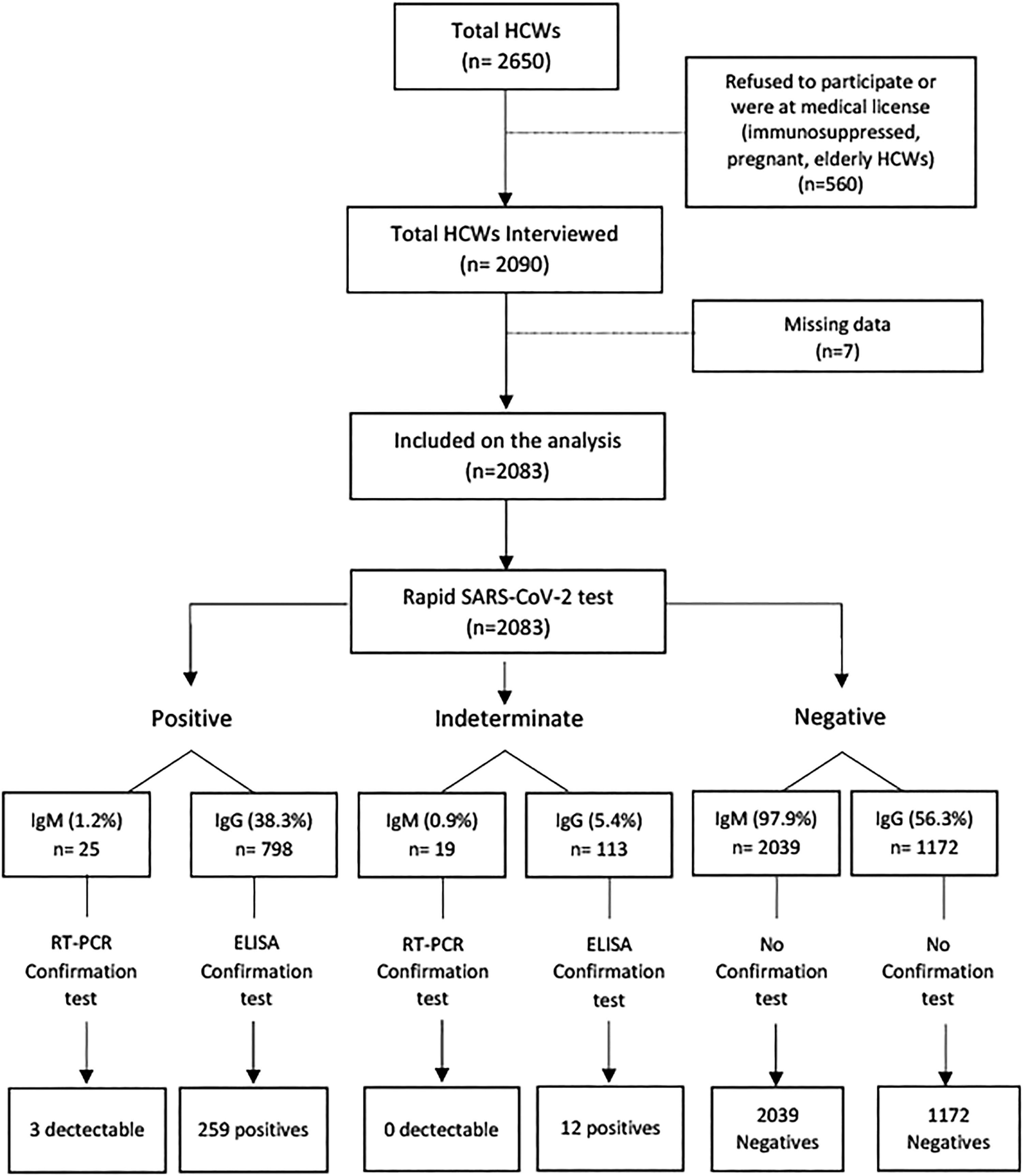
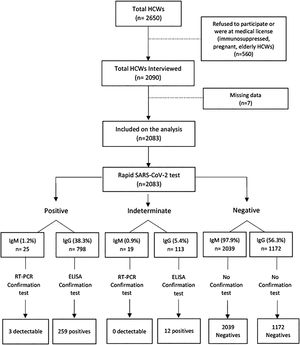
ResultsThe University Hospital has around 2,650 HCWs; 2090 were interviewed, but only 2,083 completed sociodemographic and symptom questionnaires and were included in the study. Fig. 1 summarizes the study procedures. Overall, 71.8% were women, 77.8% were self-identified as non-white, and the mean age was 41 ± 10 years. Regarding the current function, 65% were front-line health care workers (doctor, nurse, multiprofessional, nursing technician), 13.3% ancillary workers, 10.8% administrative, 6.3% of students, and 4.6% worked with diagnostic support. Most participants (72.0%) had a higher education level (graduate or postgraduate), and 153 (7.5 %) declared no religion. A total of 271 (13.0%) and 25 (1.2%) HCWs tested positive for IgG and IgM SARS-CoV-2 antibodies, respectively, and three HCWs had a detectable SARS-CoV-2 RT-PCR test, all of them had also IgG positive antibodies.
The SARS-CoV-2 incidence was 13.0% (271/2083) in the five-month period. The IPR for SARS-CoV-2 was higher among those presenting lower socioeconomic level (Table 1). In addition, there wee differences in SARS-CoV-2 incidence according to religion, with a higher incidence among Catholics and Protestants than in those who professed no religion. Table 1 depicts the difference in incidence of SARS-CoV-2 infection according to main characteristics of HCWs. There was an inverse association between the socioeconomic level, and SARS-CoV-2 incidence.
Incidence of SARS-COV-2 infection according to sociodemographic characteristics among health care workers, Salvador-Bahia.
| SARS-CoV-2 IgG ELISA | IPR (95% CI) | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Positive | Negative | |||||||
| n = 2083 | (100) | n = 271 | (13.0) | n = 1812 | (87.0) | ||||
| Age years | |||||||||
| Mean (SD) | 41 | (±10) | 42 | (±10) | 40 | (±10) | — | 0.025a | |
| Sex, n (%) | |||||||||
| Female | 1495 | (71.8) | 196 | (13.1) | 1299 | (86.9) | 1.02 | (0.80-1.32) | 0.834 |
| Male | 588 | (28.2) | 75 | (12.8) | 513 | (87.2) | 1 | ||
| Race, n (%) | |||||||||
| Black | 507 | (25.1) | 100 | (19.7) | 407 | (80.3) | 2.85 | (1.94 -4.17) | <0.001 |
| Racially Mixed | 1037 | (51.3) | 130 | (12.5) | 907 | (87.5) | 1.81 | (1.24 –2.63) | <0.001 |
| White | 448 | (22.2) | 31 | (6.9) | 417 | (93.1) | 1 | ||
| Other | 30 | (1.5) | 3 | (10.0) | 27 | (90.0) | 1.44 | (0.47 –4.46) | 0.516 |
| Education, n (%) | |||||||||
| Elementary | 60 | (2.9) | 24 | (40.0) | 36 | (60.0) | 6.05 | (4.07 –9.00) | <0.001 |
| High School | 301 | (14.5) | 99 | (32.9) | 202 | (67.1) | 4.97 | (3.70 –6.70) | <0.001 |
| Technician | 219 | (10.6) | 28 | (12.8) | 191 | (87.2) | 1.93 | (1.26 –2.96) | 0.002 |
| Graduate | 611 | (29.5) | 59 | (9.7) | 552 | (90.3) | 1.53 | (1.08 –2.17) | 0.014 |
| Postgraduate | 878 | (42.4) | 58 | (6.6) | 820 | (93.4) | 1 | ||
| Health Care Workers, n (%) | |||||||||
| Physician | 412 | (19.8) | 23 | (5.6) | 389 | (94.4) | 1.05 | (0.46 –2.40) | 0.902 |
| Nurse | 255 | (12.3) | 19 | (7.5) | 236 | (92.5) | 1.40 | (4.61 –3.26) | 0.423 |
| Diagnostic Support | 95 | (4.6) | 7 | (7.4) | 88 | (92.6) | 1.39 | (0.50 -3.83) | 0.523 |
| Multiprofessionalb | 162 | (7.8) | 15 | (9.3) | 147 | (90.7) | 1.75 | (0.73 –4.16) | 0.200 |
| Nursing technician | 522 | (25.1) | 61 | (11.7) | 461 | (88.3) | 2.20 | (1.03 –4.70) | 0.031 |
| Administrative | 225 | (10.8) | 27 | (12.0) | 198 | (88.0) | 2.26 | (1.01 –5.05) | 0.037 |
| Ancillary workersc | 277 | (13.3) | 112 | (40.4) | 165 | (59.6) | 7.62 | (3.65 –15.90) | <0.001 |
| Student | 132 | (6.3) | 7 | (5.3) | 125 | (94.7) | 1 | ||
| Family Income, n (%) | |||||||||
| <3 minimum wages | 348 | (17.6) | 97 | (27.9) | 251 | (72.1) | 3.88 | (2.96 –5.09) | <0.001 |
| 3-5 minimum wages | 532 | (26.9) | 66 | (12.4) | 466 | (87.6) | 1.72 | (1.27 –2.35) | <0.001 |
| >5 minimum wages | 1099 | (55.5) | 79 | (7.2) | 1020 | (92.8) | 1 | ||
| Religion, n (%) | |||||||||
| Catholic | 1047 | (51.4) | 143 | (13.7) | 904 | (86.3) | 2.32 | (1.21 –4.46) | 0.006 |
| Protestant | 838 | (41.1) | 107 | (12.8) | 731 | (87.2) | 2.17 | (1.12 –4.19) | 0.014 |
| Without Religion | 153 | (7.5) | 9 | (5.9) | 144 | (94.1) | 1 | ||
* Column percentage; **Line percentage.
The logistic regression model (X2 (8) = 4.612, p > 0.798), showed a good fit for the data, which explained 16.0%. Nagelkerke R2 of the variance in COVID-19 correctly classified 88.0% of cases.19 The regression analysis, completed based on 1,887 observations, was performed to ascertain the association between HCWs functional group, education, race, family income, and religion and COVID-19 incidence.
Ancillary workers were almost 5-fold more likely to have COVID-19 than medical students (OR: 4.96, 95% CI: 1.95–12.63). Lower level, elementary education (OR: 2.91, CI 95: 1.19–7.13), and high level of schooling (OR: 2.89 95% CI: 1.61–5.18), remained independently associated with an increased likelihood of presenting a COVID-19 positive test. In addition, Catholics (OR: 2.16, 95% CI: 1.01–4.62) were 2-fold more likely to have a positive COVID-19 test than those without religion (Table 2).
Binary logistic regression with SARS-COV-2 as the outcome among 1,887 health care workers, Salvador - Bahia.
Abbreviations: OR (Odds Ratio); CI (Confidence Interval).
Table 3 displays the main clinical characteristics reported by the study participants. Ageusia (OR: 7.41 95% CI: 6.15–8.94) and anosmia (OR: 8.51 95% CI: 7.09–10.22) were the most frequent symptoms associated with COVID-19 incidence.
Incidence (%) of SARS-CoV-2 infection according to signs and symptoms of Covid-19 among health care workers, Salvador-Bahia.
| Sign/Symptom | SARS-COV-2 IgG ELISA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Positive | Negative | IPR (95% CI) | P-value | ||||||
| n = 2083 | (100) * | n = 271 | (13.0) ⁎⁎ | n = 1812 | (87.0) ⁎⁎ | |||||
| At least one sign or symptom | 1434 | (68.8) | 225 | (15.7) | 1209 | (84.3) | 2.21 | (1.63 – | 3.00) | <0.001 |
| Abdominal pain | 316 | (15.2) | 47 | (14.9) | 269 | (85.1) | 1.17 | (0.88 – | 1.57) | 0.286 |
| Ageusia | 178 | (8.6) | 111 | (62.4) | 67 | (37.6) | 7.41 | (6.15 – | 8.94) | <0.001 |
| Anosmia | 173 | (8.3) | 118 | (68.2) | 55 | (31.8) | 8.51 | (7.09 – | 10.22) | <0.001 |
| Arthralgia | 336 | (16.1) | 75 | (22.3) | 261 | (77.7) | 1.99 | (1.56 – | 2.52) | <0.001 |
| Ataxia | 80 | (3.8) | 23 | (28.8) | 57 | (71.3) | 2.32 | (1.61 – | 3.34) | <0.001 |
| Cough | 488 | (23.4) | 100 | (20.5) | 388 | (79.5) | 1.91 | (1.52 – | 2.39) | <0.001 |
| Diarrhea | 446 | (21.4) | 66 | (14.8) | 380 | (85.2) | 1.18 | (0.91 – | 1.52) | 0.205 |
| Dizziness | 243 | (11.7) | 49 | (20.2) | 194 | (79.8) | 1.67 | (1.26 – | 2.21) | <0.001 |
| Dyspnea | 162 | (7.8) | 43 | (26.5) | 119 | (73.5) | 2.23 | (1.68 – | 2.97) | <0.001 |
| Expectoration | 154 | (7.4) | 39 | (25.3) | 115 | (74.7) | 2.11 | (1.57 – | 2.83) | <0.001 |
| Fatigue | 506 | (24.3) | 102 | (20.2) | 404 | (79.8) | 1.88 | (1.50 – | 2.35) | <0.001 |
| Fever | 237 | (11.4) | 75 | (31.6) | 162 | (68.4) | 2.98 | (2.37 – | 3.75) | <0.001 |
| Headache | 1047 | (50.3) | 162 | (15.5) | 885 | (84.5) | 1.47 | (1.17 – | 1.84) | <0.001 |
| Hypoacusis | 41 | (2.0) | 9 | (22.0) | 32 | (78.0) | 1.08 | (0.65 – | 1.77) | 0.085 |
| Hyporexia | 243 | (11.7) | 87 | (35.8) | 156 | (64.2) | 3.58 | (2.88 – | 4.45) | <0.001 |
| Mental confusion | 30 | (1.4) | 11 | (36.7) | 19 | (63.3) | 2.89 | (1.78 – | 4.69) | <0.001 |
| Myalgia | 498 | (23.9) | 106 | (21.3) | 392 | (78.7) | 2.05 | (1.64 – | 2.55) | <0.001 |
| Nausea | 345 | (16.6) | 67 | (19.4) | 278 | (80.6) | 1.65 | (1.28 – | 2.12) | <0.001 |
| Odynophagia | 596 | (28.6) | 74 | (12.4) | 522 | (87.6) | 1.08 | (0.65 – | 1.77) | 0.763 |
| Palpitations | 231 | (11.1) | 38 | (16.5) | 193 | (83.5) | 1.30 | (0.95 – | 1.79) | 0.099 |
| Paresis | 80 | (3.8) | 24 | (30.0) | 56 | (70.0) | 2.43 | (1.70 – | 3.46) | <0.001 |
| Paresthesia | 150 | (7.2) | 29 | (19.3) | 121 | (80.7) | 1.54 | (1.09 – | 2.18) | 0.016 |
| Skin disorder | 87 | (4.2) | 11 | (12.6) | 76 | (87.4) | 0.97 | (0.55 – | 1.70) | 0.917 |
| Skin rash | 39 | (1.9) | 3 | (7.7) | 36 | (92.3) | 0.58 | (0.19 – | 1.75) | 0.319 |
| Sneezing | 788 | (37.8) | 121 | (15.4) | 667 | (84.6) | 1.32 | (1.06 – | 1.66) | 0.013 |
| Thoracic pain | 182 | (8.7) | 44 | (24.2) | 138 | (75.8) | 2.02 | (1.52 – | 2.69) | <0.001 |
| Tinnitus | 98 | (4.7) | 19 | (19.4) | 79 | (80.6) | 1.52 | (1.00 – | 2.32) | 0.054 |
| Urinary incontinence | 42 | (2.0) | 13 | (31.0) | 29 | (69.0) | 2.44 | (1.53 – | 3.90) | <0.001 |
| Urinary retention | 6 | (0.3) | 3 | (50.0) | 3 | (50.0) | 3.87 | (1.72 – | 8.69) | 0.006 |
| Urinary urgency | 59 | (2.8) | 8 | (13.6) | 51 | (86.4) | 1.04 | (0.54 – | 2.00) | 0.848 |
| Visual disturbance | 50 | (2.4) | 7 | (14.0) | 43 | (86.0) | 1.08 | (0.53 – | 2.16) | 0.833 |
| Vomiting | 78 | (3.7) | 15 | (19.2) | 63 | (80.8) | 1.50 | (0.94 – | 2.40) | 0.096 |
This study describes the main characteristics of 2,083 HCWs at a tertiary hospital in Salvador-Brazil, who were tested for SARS-CoV-2 antibodies. A total of 271 (13.0%) HCWs tested positive for SARS-CoV-2 antibodies, and the sociodemographic markers of social vulnerability were predictive of a higher likelihood of infection by SARS-CoV-2. In addition, Catholic religion, and clinical findings of anosmia, and/or ageusia were strongly associated to SARS-CoV-2 infection.
Socially vulnerable HCWs showed the highest COVID-19 incidence, reflecting similar pattern observed among non-HCW population.20,21 We infer that factors like low income, informal work status, low level of education, and black or mixed race, are markers of social vulnerability and are indirect markers of use of mass public transportation, poor access to basic services and probably inadequate use of personal protection equipment. We also found that having no religion was associated with lower risk of COVID-19 infection. Some religious practices can contribute to the spread of COVID-19 due to extended transmission during communal religious prayers and large attendance at religious gatherings and festivals.22,23 This association was poorly studied, especially during lockdown periods, in a retrospective study people declaring no religion had the lowest risk of COVID-19 related death, before and after lockdowns.23
The detected associations between social vulnerability and a higher risk of COVID-19 demonstrate that the risk of infection by SARS-CoV-2 is not only determined by the exposure. Non-white race, lower educational level and lower family income are strongly associated with a higher risk of infection, in a HCW population. In this study, it clearly indicates that the risk of COVID-19 in a hospital is not a direct consequence of a higher exposure to infected patients but is strongly influenced by the HCW´s educational level, and social position. Probably these characteristics are a proxy of lower adherence to preventive actions, like masks use, social distancing and hands hygiene.24
Social disparities were previously described in a Brazilian population-based study, showing that black and mixed individuals had infection rates 81% and 45% higher than whites, with a higher percentage of deaths occurring in primary-care or isolated emergency-care units and predominantly in public institutions, as a reflex of barriers to healthcare access. A positive gradient was found for all indicators of socioeconomic status and increases in disparities (denoted by less education, more household crowding, lower income, and higher concentration of subnormal areas) were associated with higher mortality rates.25 The same pattern seems to be repeated in other countries with different sociodemographic, cultural and health system characteristics, like the United States and the United Kingdom.25-26 Indirectly, the higher rates of infection in vulnerable populations, contributes to increase on contagion, saturation of public health systems or difficulty of access to private health systems and consequently higher mortality.
When the contemporary Brazilian epidemiological report13 is compared to the dates in which our study was carried out, we observe similar proportions of infected nursing technicians, nurses, and doctors, with nursing technicians being the most affected group. This indicates that regardless of the type of service, whether this hospital is a referral for COVID-19 or not, professionals with a lower degree of education could be at higher risk not only due to the greater exposure, but also due to educational factors and socioeconomic limitations. In addition, it was not possible to determine if the contagion occurred during the intra-hospital service or in the community. The low frequency of HCWs with a detectable RT-PCR was expected, as all interviewed participants were on duty within the hospital and were asymptomatic at the moment of testing.
The initial reports described fever and cough as the most frequent symptoms related to COVID-19.27,28,29 However, following the increasing number of reports on the clinical characteristics of the disease, ageusia and anosmia became common symptoms, and highly suggestive of COVID-19.19 In the current study, anosmia and ageusia were the most frequent symptoms presented by infected HCWs, and their presences were significantly associated with COVID-19 incidence. Usually such symptoms are not accompanied by nasal obstruction or rhinitis symptoms; this probably occurs due to direct damage caused by the virus on the olfactory and gustatory receptors 30 or due to altered mucosal immune response in the upper airways.31Table 3 shows that COVID-19 related symptoms, ageusia and anosmia, were strongly associated with COVID-19 positivity.
Our study has some limitations: the cross-sectional design does not allow to determine causality. However, we investigated all active HCWs of a tertiary hospital, which makes the detected associations consistent for the target population and minimizes selection bias. Given the circumstances and the study design, we were unable to adjust for factors such as community and occupational exposures, including the frequency of direct contact with COVID-19 patients or compliance to PPE (personal protective equipment) use. Even with the use of two tests to determine the serological status, there is a likelihood of false positive and false negative results. Some patients also are not able to develop a detectable production of antibodies against SARS-CoV-2, as we were not able to predict the exact number of cases on immunological window, which could also indicate a lower than actual seroprevalence rate. However, the testing approach we used (two screening tests, ELISA and PCR when necessary) was robust enough to provide a safe estimate of seroprevalence rate.
The findings from this large sample reinforce the need of a better understanding of the factors associated with an increased risk of COVID-19 in the HCW population. In conclusion, we observed that among HCWs at a tertiary hospital, markers of social vulnerability like: low mean family income, lower level of schooling, ancillary work, or being black had a significantly higher likelihood of testing positive for SARS-CoV-2 antibodies, those HCWs characteristics seems to be a more important risk factor for COVID-19 than the occupational exposure to infected patients. This knowledge can provide important insights for development and implementation of preventive strategies aiming at minimizing the risk of infection by SARS-CoV-2 in similar institutions, especially in a continental-wide country, like Brazil.
Funding“This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001”. The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The last author is a CNPq researcher (process number: 311095/2020-8).