The aim of this study was to monitor rotavirus (RV) infections in adults >18 years with acute gastroenteritis during 2004–2011 national Brazilian RV surveillance. In addition, to characterize the RV group A (RVA) strains in order to gain insight into the supposed vaccine selective pressure imposed to Brazilian children population.
MethodsA total of 2102 convenient fecal specimens were investigated by ELISA, PAGE, and RT-PCR.
ResultsRV was detected in 203 (9.6%) of 2102 specimens, and showed a marked peak of detection in September. RVA infection was detected in 9.4% (197/2102) and RV group C (RVC) in 0.3% (6/2102). The most frequent genotypes detected in 2004 and 2005 were G9P[8] (38.5%; 5/13) and G1P[8] (54.5%; 6/11), respectively. The dominant genotype identified from 2006 to 2011 was G2P[4] (64.4%; 116/180). Detection rate varied during the 8-year period of the study from 0.7% to 12.9%.
ConclusionThe high detection rate of G2P[4] in adults provides further evidence that its dominance reflects the seasonality of RVA strains instead of the supposed selective advantage created by vaccination program. It also can be suggested that adult infections may serve as a reservoir to maintain RVA strains in childhood gastroenteritis. Considering the detection rate, the evident reduction of RVA frequency observed in children after vaccine introduction was not present in adults.
Rotaviruses (RV) are double-stranded RNA (dsRNA) viruses comprising the genus Rotavirus in the family Reoviridae.1 Three of the seven identified groups of RV infect humans. Group A rotavirus (RVA), the most common cause of diarrhea in infants and young children throughout the world, accounts for the large percentage of pediatric hospitalizations.2 As a pathogen of adults, RVA has frequently been overlooked.3,4 Some authors have reported RVA cases in elderly and immune compromised individuals.5,6 Both VP7 and VP4, outer proteins layer, are used to further categorize RVA into G (Glycoprotein) and P (Protease) genotypes. The most common G types detected are G1, G2, G3, G4 and G9, while in P typing P[4], P[6] and P[8] are the most frequently isolated.7,8
RVA infection and circulating genotypes in children in Brazil have been well characterized.9,10 Nevertheless, limited information is available on the genotypes infecting adults.8,11 In addition, a high prevalence of G2P[4] was lately reported in Brazil and linked with universal RVA vaccination program using a G1P[8] live oral vaccine suggesting that this monovalent vaccine possibly created conditions in which G2P[4] could acquire selective advantage over P[8] genotypes.12 Nevertheless, a temporal periodicity, within ≈10 year cyclic pattern of G2P[4] has also been observed in Brazil,13 and should have been considered as an alternative explanation to the increased detection of this genotype since 2006. The molecular characterization of RVA genotypes circulating in adults could help to solve this question.
Group B (RVB) and group C (RVC) rotavirus are morphologically identical to RVA, but their dsRNA presents distinct migration patterns on polyacrylamide gels and they are not detected by common enzyme immunoassays (EIAs) for RVA.14 RVB has been associated with outbreaks of diarrhea in adults in China, India, and Bangladesh.15,16 RVC has been associated with either sporadic diarrheal illness or limited outbreaks of illness in various settings, 15,17,18 including Brazil.19–22
The aim of this work was to monitor RV infections in adults >18 years old with acute gastroenteritis during 2004–2011 national Brazilian RV surveillance. Additionally, to characterize the RVA strains (G- and P-type) circulating in the adult community during this study period in order to gain insight into the supposed vaccine selective pressure imposed to Brazilian children population.
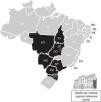
Material and methodsFecal samplesThis is a retrospective and descriptive study conducted from 2004 to 2011 with convenient sampling from surveillance specimen collected from adults >18 years old presenting acute gastroenteritis. Stool samples from patients with acute gastroenteritis were sent to the Enteric Diseases Laboratory of the Adolfo Lutz Institute, regional reference center for rotavirus surveillance, Ministry of Health – Brazil, and a member of Acute Diarrhea Disease Monitoring Program, State Department of Health. The aim of this program is the early detection of outbreaks of diarrhea with national scope. In this study, clinical samples from the following States were used: São Paulo (SP), Mato Grosso (MT), Mato Grosso do Sul (MS), Paraná (PR), Tocantins (TO), Goiás (GO), Santa Catarina (SC), and the Federal District (FD) (Fig. 1).
The states highlighted in black collected stool samples from patients with acute gastroenteritis, screened for rotavirus group A infection and sent to the Enteric Diseases Laboratory of the Adolfo Lutz Institute. Adolfo Lutz Institute is Regional Reference Center for rotavirus surveillance and a member of Acute Diarrhea Disease Monitoring Program with national scope.
A total of 2102 stool samples were tested. RVA was detected using commercial immunoenzymatic assays (Premier™ Rotaclone®, Meridian Bioscience, Inc., USA; or RIDASCREEN® Rotavirus, RBiopharm AG, Darmstadt, Germany) performed according to the manufacturer's instructions. RVA ELISA negative samples were tested further for the presence of RVC by polyacrylamide gel electrophoresis (SDS–PAGE) according to standard procedures,23 and confirmed by RT-PCR assay.14
RNA extraction and RT-PCRRVA and RVC dsRNA was extracted directly from stools using Trizol® reagent (Invitrogen, Carlsbad, CA, USA),24 according to the manufacturer's instructions or by QIAamp® Viral RNA Mini kit (Qiagen, Valencia, CA), and stored at −70°C. The reverse transcriptase polymerase chain reactions (RT-PCR) for RVA VP7 and VP4 genes were performed according to the protocols previously described.25,26 RT-PCR protocol used to amplify a fragment of the RVC VP6 gene was described by Gouvea et al.14
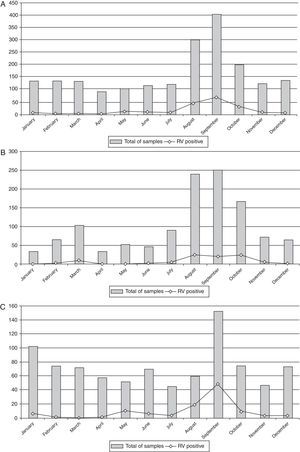
ResultsRV was detected in 203 (9.6%) of 2102 specimens collected from adults (Table 1). RV infection was found predominantly in the winter and in drier months, with peak in September (Fig. 2A). Temporal analysis of RV infection allowed the identification of two sections. The first section, between 2004 and 2007, did not reveal clear peak of RV detection, but rather a tendency of RV detection among August, September and, October (Fig. 2B). The second section, between 2008 and 2011, showed a marked peak of RV detection occurring in September (Fig. 2C). RVA infection was detected in 9.4% of the samples (197/2102) and RVC in 0.3% (6/2102). RVC cases were detected only in 2008 (3.4%; 6/178) (Table 1).
Rotavirus genotyping results from adults ≥18 years old presenting acute gastroenteritis, Brazil, 2004–2011.
| Rotavirus genotype | Year | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2004% (No.) | 2005% (No.) | 2006% (No.) | 2007% (No.) | 2008% (No.) | 2009% (No.) | 2010% (No.) | 2011% (No.) | Total % (No.) | |
| G1P[4] | – | – | 1.9% (1) | 25.0% (2) | – | – | – | – | 1.7% (3) |
| G1P[8] | 30.7% (4) | 54.5% (6) | 7.7% (4) | – | – | – | – | – | 7.8% (14) |
| G2P[4] | – | – | 59.7% (31) | 62.5% (5) | 77.3% (17) | – | 90.4% (57) | 60% (6) | 64.5% (116) |
| G3P[8] | – | 9.1% (1) | – | – | – | – | 4.8% (3) | – | 2.2% (4) |
| G9P[8] | 38.5% (5) | 9.1% (1) | 9.6% (5) | – | – | – | – | 10% (1) | 6.7% (12) |
| G2+G9P[NT] | – | – | 3.8% (2) | – | – | – | – | – | 1.1% (2) |
| G2+G3P[4]+[8] | – | – | – | – | – | – | 1.6% (1) | – | 0.5% (1) |
| G9P[NT] | 15.4% (2) | – | – | – | – | – | 1.6% (1) | – | 1.7% (3) |
| G1P[NT] | – | – | 5.8% (3) | – | – | – | – | – | 1.7% (3) |
| G2P[NT] | – | – | 1.9% (1) | – | 4.5% (1) | – | – | 20% (2) | 2.2% (4) |
| GNTP[8] | 7.7% (1) | – | – | – | – | – | – | – | 0.5% (1) |
| GNTP[4] | – | – | – | – | – | – | – | 10% (1) | 0.5% (1) |
| GNTP[NT] | 7.7% (1) | 27.3% (3) | 9.6% (5) | 12.5% (1) | 18.2% (4) | 100% (1) | 1.6% (1) | – | 8.9% (16) |
| Total of genotyped samples | 68.4% (13) | 91.7% (11) | 96.3% (52) | 80.0% (8) | 95.6% (22) | 100% (1) | 92.6% (63) | 100% (10) | 91.4% (180) |
| RT-PCR negative | 31.6% (6) | 8.3% (1) | 3.7% (2) | 20.0% (2) | 4.4% (1) | – | 7.4% (5) | – | 8.6% (17) |
| Total RVA ELISA positive | 7.8% (19) | 4.3% (12) | 11.2% (54) | 4.6% (10) | 12.9% (23) | 0.7% (1) | 20.0% (68) | 4.6% (10) | 9.4% (197) |
| RVC | – | – | – | – | 3.4% (6) | – | – | – | 0.3% (6) |
| Total of samples | 244 | 277 | 484 | 217 | 178 | 146 | 339 | 217 | 2102 |
NT, non-typed.
The median age for patients infected with the RVA was 41.5 years old, 63.4% being females. The median age for patients infected with the RVC was 33.3 years old, 66.7% being females. The most frequent genotypes detected in 2004 and 2005 were G9P[8] (38.5%; 5/13) and G1P[8] (54.5%; 6/11), respectively. The dominant genotype identified from 2006 to 2011 was G2P[4] (64.4%; 116/180). Detection rate varied during the 8-year period of the study: 7.8% (19/244) in 2004, 4.3% (12/277) in 2005, 11.2% (54/484) in 2006, 4.6% (10/217) in 2007, 12.9% (23/178) in 2008, 0.7% (1/146) in 2009, 20.0% (68/339) in 2010, and 4.6% (10/217) in 2011 (Table 1).
DiscussionThis study was designed to investigate the frequency of RV infection in adults ≥18 years old with acute gastroenteritis and identified the RVA strains in order to investigate possible vaccine impact on the Brazilian pediatric population. In healthy adults, infection causes few or mild symptoms and RV infection is diagnosed via differential diagnosis. However, in immune compromised patients, infection can be severe and persistent.27
RVA spread occurs mainly through the fecal–oral route, with transmission to adults probably due to caring for sick children or ingestion of contaminated food or water.4 The peak in RV infections occurred in winter months as it is usually observed in most of the world28–30; and also mirror the winter RV seasonality of infection observed in pediatric patients.8,31–34
In tropical and subtropical settings, the peak of RV infections occurs in cool dry seasons.28 Strict winter seasonality was also common in the Americas,29 including Brazil's southern area,30 although sporadic RV cases and outbreaks can occur all along the year. However, a particular event was observed. The RV detection rate is usually concentrated between June and August in Brazil8,31–34; nevertheless, the frequency of RV detection in this study peaked in September, month that marks the beginning of the rainy season. The seasonal patterns and climatic sensitivities of many infectious diseases are well known35; and global warming is one of the environmental changes now under way. It can be suggested that this climatic variation could promote a range of impacts upon the occurrence of RV infection in the Brazilian population.
The frequency of RVA infection in adults detected in this work (9.4%) was lower than that observed in studies carried out in Japan (14%),3 United States (18%)36 and Paraguay (17.3%),37 but was similar to the frequency found in China (9%).38 A previous study conducted in Brazil showed that RVA rate among adults was 7.1%,8 comparable to the incidence observed in India (7%).39 The overall percentage of RVA detected among children in Brazil has been much higher (∼30%) than that found in adults.8,9 A significant reduction in the frequency of RVA detection in children with gastroenteritis was observed in Brazil after the RVA vaccine introduction40; nonetheless, the present study showed that this decrease was not evident in adult inhabitants. On the other hand, Anderson et al.41 showed that the prevalence of RVA among adults declined from 4.35% in 2006–2007 (prepediatric impact era) to 2.24% in 2008–2010 (pediatric impact era) in USA, suggesting an indirect protection of adults from RVA by pediatric RVA vaccination.
Considering RVA annual frequency, a low rate of RVA infection was observed in 2009. A similar lack of a RVA season in Brazil during 2009 has been previously described.10,42,43 According to the National Meteorology Institute (INMET) records, during the year of 2009, Brazil experienced an atypical raining winter season with higher average rainfall record due to the El Niño phenomenon (http://www.inmet.gov.br). Therefore, this climatic condition could have been involved in the low detection frequency observed.42
A variety of RVA genotypes among adults was described all over the world.11,14,44–47 Throughout the study period, G2P[4] was the most frequent strain in adults (64.5%). G1P[8] and G9P[8] were the most frequent types in 2004–2005, matching to the results of numerous studies focusing RVA G type distribution in many countries, including Brazil.8,48 However these strains were displaced by G2P[4] during the 2006 season. Recently, a high prevalence of G2P[4] in children was reported in Brazil and linked to universal RV vaccination program using a G1P[8] live oral rotavirus vaccine.12 Nevertheless, the high detection rate of G2P[4] in adults provides further evidence that its predominance reflects the epidemic cycle of RVA strains instead of the supposed selective advantage created by the monovalent G1P[8] vaccination program. On the other hand, it also can be suggested that adult infections may serve as a reservoir to maintain RVA strains in childhood gastroenteritis. In addition, the same strains detected in adults during the study period were known to be circulating in children according to Laboratory records.
G4P[8] strain is one of the most common combinations found in humans worldwide,2 however, it was not detected in this study. At a global level, the weighted prevalence of G4 decreased slightly during 1996–2007.49 The seasonal shifts of RVA strains is a possible mechanism used by the virus to evade herd immunity acquired by previous infections, and thus, ultimately persist in the human population.50 The changing pattern of circulating RVA types associated with greatly reduced diversity of strains may have important implications for the evolution of RVA strains.10
It is worth mentioning that RVA genotyping by RT-PCR presents some limitations. RT-PCR is the method of choice for typing RVA and is regarded as the gold standard. However, the accumulation of point mutations at primer binding sites has been linked with mistyping or failure to identify genotype correctly. Regularly validation of PCR results with sequencing is important,51 especially in mixed RVA infections. In addition, in the present study RVA positive samples could not be genotyped by multiplex RT-PCR. Unfortunately, non-typeable RVA strains have been reported in almost every epidemiological survey around the world, regardless of the methodology employed.52
RVC infection in adults has received negligible attention. Only a few studies documented the RVC occurrence in adults, most of them based on serological evidence.16,53–55 In Brazil, RVC was previously detected both in sporadic cases and outbreaks affecting adults.20–22 The RVC detection rate observed in this work (0.3%) was lower than that observed in a study carried out with adults in Sweden (∼1%).56 It is noted that the RVC incidence and associated disease remain unclear once sensitive tests for its detection are not available to clinical laboratories. The diagnosis is difficult since most of the EIA assays do not recognize the RVC specific VP6 antigen. The RT-PCR using RVC specific primer is a convenient option57; however, it has not been used widely due to the large costs for routine surveillance. The PAGE analysis of dsRNA requires the presence of 108–1010 viral particles/ml for a positive result57; nevertheless, this method has sufficient sensitivity to detect RVC with low costs.42
In conclusion, this study adds further evidence that RV is a minor cause of acute adult gastroenteritis in Brazil. On the other hand, contributes significantly to the understanding of RV infections in mature population. The results also suggest that adults are susceptible to the same types of RVA at the same season of the year as children. It is still unknown whether the burden of RVA in adults will remain unchanged subsequently after widespread impact of RVA vaccination observed in children.41 The finding of different genotypes in the adult population highlights the need for continuing surveillance in both pediatric and mature populations, to monitor the suitability of vaccines and to correlate vaccine efficacy with continuing virus evolution.4 The dynamics between pediatric and adult RVA infection remains unclear, and adult infections may serve as another source of RVA genomic diversity.
Ethical approvalThis study was carried out in accordance with the Declaration of Helsinki as revised in 2000, and approved by the Ethics Committee of the Adolfo Lutz Institute, São Paulo. Study participants were not required to provide informed consent as this study was considered by the Ethics Committee to be part of routine surveillance activities.
Author contributionsAL and MCSTT conceived and designed the study; AL, AC, SGM, RCCC acquired the data, performed the immunoenzymatic assays, and the molecular typing of the strains; AL analyzed the data and wrote the manuscript; AC, SGM, RCCC and MCSTT critically revised the manuscript. All authors read and approved the final version. AL and MCSTT are guarantors of the paper.
Conflicts of interestThe authors declare no conflicts of interest.
We thank the Enteric Diseases Laboratory of Adolfo Lutz Institute staff: Adeline M. Fernandes, Ana Paula T. Santos, Camila Goto, Carolina J. Gill, Cibele D. Ribeiro, Fernanda F. Costa, Heloisa R. Vieira, Raquel Guiducci and Samira J. Calux for laboratorial analysis assistance; Antonio Erculiani Junior and Sirlene Henrique Rodrigues Silva for technical assistance. We are grateful to Centers for Surveillance (CVE), São Paulo State Health Department; Public Health Laboratories (LACENs) and, CGLAB/DEVEP/SVS/Ministry of Health, Brasília for assistance in samples collection and epidemiological data. We thank Camila Buoro Auler for critical language review.