Fluoroquinolones are the backbone of multidrug resistant tuberculosis treatment regimens. Despite the high burden of multidrug resistant tuberculosis in the country, little is known about drug resistance patterns, prevalence, and predictors of fluoroquinolones resistance among multidrug resistant tuberculosis patients from Pakistan.
ObjectiveTo evaluate drug resistance patterns, prevalence, and predictors of fluoroquinolones resistance in multidrug resistant tuberculosis patients.
MethodsThis was a cross-sectional study conducted at a programmatic management unit of drug resistant tuberculosis, Lady Reading Hospital Peshawar, Pakistan. Two hundred and forty-three newly diagnosed multidrug resistant tuberculosis patients consecutively enrolled for treatment at study site from January 1, 2012 to July 28, 2013 were included in the study. A standardized data collection form was used to collect patients’ socio-demographic, microbiological, and clinical data. SPSS 16 was used for data analysis.
ResultsHigh degree of drug resistance (median 5 drugs, range 2–8) was observed. High proportion of patients was resistant to all five first-line anti-tuberculosis drugs (62.6%), and more than half were resistant to second line drugs (55.1%). The majority of the patients were ofloxacin resistant (52.7%). Upon multivariate analysis previous tuberculosis treatment at private (OR=1.953, p=0.034) and public private mix (OR=2.824, p=0.046) sectors were predictors of ofloxacin resistance.
ConclusionThe high degree of drug resistance observed, particularly to fluoroquinolones, is alarming. We recommend the adoption of more restrictive policies to control non-prescription sale of fluoroquinolones, its rational use by physicians, and training doctors in both private and public–private mix sectors to prevent further increase in fluoroquinolones resistant Mycobacterium tuberculosis strains.
Multidrug resistant tuberculosis (MDR-TB) defined as resistance to both isoniazid (H, INH) and rifampicin (R, Rif) is a major barrier to achieve successful TB control. Both INH and Rif are the most effective anti-TB drugs and the backbone of first line anti-TB treatment. Resistance to INH and Rif leads treatment with less potent, more toxic and expensive second-line anti-TB drugs (SLD). Fluoroquinolones (FQ) — broad spectrum antibiotics — have been shown to be useful in TB treatment — have been used in TB care since 1984, and have become integral part of drug resistant TB treatment regimens.1 Various studies have reported positive associations between FQ resistance and poor treatment outcomes in MDR-TB.2–5 Unfortunately, Pakistan in addition to be the 5th highest country of TB burden also harbors the largest population of MDR-TB patients in Eastern Mediterranean Region of WHO. It is estimated that 9900 (95% confidence interval [CI]: 6400–13,300) new MDR-TB cases emerged in Pakistan in 2013 with an estimated proportion of 4.3% (95% CI: 2.8–5.7) of new cases and 19% (95% CI: 14–25) of previously treated TB cases.6 This situation is further worsened by reportedly increased FQ resistance in drug resistant TB in the country.7,8 Few studies from Pakistan have evaluated the prevalence of FQ resistance in MDR-TB patients,7–10 but to the best of our knowledge none has evaluated predictors of FQ resistance in MDR-TB patients. Therefore, the present study was conducted with the aim to evaluate patterns of drug resistance, prevalence, and predictors of FQ resistance among MDR-TB patients.
Materials and methodsStudy design and settingsThis was a cross-sectional study conducted at a programmatic management unit of drug resistant TB (PMDT), Lady Reading Hospital (LRH) Peshawar, Pakistan. At the time of study initiation, PMDT unit LRH was the only center in Khyber Pukhtoonkhwa (one of the four provinces of Pakistan) where drug resistant TB was treated. All MDR-TB patients consecutively enrolled for treatment at the study site from January 1, 2012 to July 28, 2013 were included in the study. Since October 1, 2012 data were collected prospectively while before October 1, 2012 data were collected retrospectively. Patients with mono, poly, and extensive drug resistant TB (XDR-TB) and/or history of previous treatment of drug resistant TB were excluded.
Drug resistant TB (DR-TB) suspects referred to the study site were initially evaluated with two sputum samples for acid fast bacilli (AFB) by direct sputum smear microscopy using Ziehl Neelsen staining method and GeneXpert System's MTB/Rif (Mycobacterium tuberculosis/rifampicin). Upon positive smear microscopy and GeneXpert System's MTB/Rif, sputum samples were sent to Aga Khan University Hospital Laboratory for sputum culture and DST. Susceptibility testing for isoniazid (H), rifampicin (R), ethambutol (E), streptomycin (S), ofloxacin (Ofx), amikacin (Am), kanamycin (Km), ethionamide (Eto), and capreomycin (Cm) was conducted by using agar proportion method on enriched Middle brook 7H10 medium (BBL, Beckton Dickinson). Pyrazinamide (PZA, Z) susceptibility test was carried out by using BACTEC Mycobacterial Growth Indicator Tube (Becton Dickinson Diagnostics, Sparks, MD, USA) in accordance with manufacturer's instructions. A standardized data collection form was used to collect patients’ socio-demographic, microbiological, and clinical data.
Statistical analysisStatistical Package for Social Sciences (SPSS 16) was used for data analysis. Means and standard deviations were calculated for continuous variables, whereas categorical variable were presented as frequencies and percentages. Chi-squared test was used to observe association between categorical variables. Multivariate logistic regression analysis with Wald statistical criteria using the backward elimination method was used to obtain a final model describing the predictors FQ resistance. A p-value of <0.05 was considered statistically significant. Relevant variables with p-value <0.2 in univariate analysis were included in multivariate analysis. We checked correlation among variables entered in multivariate analysis.
Ethical approvalThe study was approved by the Research and Ethics Committee of Postgraduate Medical Institute, Peshawar, Pakistan. Prior to beginning of the study, written consent was taken from patients who were able to do so. In case of illiteracy the purpose of study was explained to the patients in their native language, and next a kin or treatment supporter gave written consent on behalf of the patient. This consent procedure was approved by Research and Ethics Committee.
ResultsDuring the study period a total of 289 drug resistant TB patients were enrolled at the study site. A total of 46 patients were excluded: 29 with drug resistant TB other than MDR-TB (10 with mono-drug resistant TB, six with poly-drug resistant, and 13 with extensive drug resistant) and 17 with history of previous treatment for MDR-TB (Fig. 1).
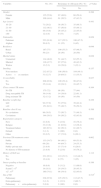
Mean age of patients was 30.4±14.4 years. Socio-demographic, baseline clinical and microbiological characteristics of 243 patients included in the study are given in (Table 1).
Patients’ socio-demographic, baseline clinical characteristics and ofloxacin resistance.
| Variables | No. (%) | Resistance to ofloxacin (No. %) | p-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Gender | 0.288 | |||
| Female | 135 (55.6) | 67 (49.6) | 68 (50.4) | |
| Male | 108 (44.4) | 61 (56.5) | 47 (43.5) | |
| Age (years) | 0.441 | |||
| 10–20 | 71 (29.2) | 36 (50.7) | 35 (49.3) | |
| 21–40 | 112 (46.1) | 57 (50.9) | 55 (49.1) | |
| 41–60 | 48 (19.8) | 26 (45.2) | 22 (45.8) | |
| >60 | 12 (4.9) | 9 (75) | 3 (25) | |
| Nationality | 0.828 | |||
| Pakistani | 223 (91.8) | 117 (52.5) | 106 (47.5) | |
| Afghan | 20 (8.2) | 11 (55) | 9 (45) | |
| Residence | 0.648 | |||
| Rural | 187 (77) | 100 (53.5) | 87 (46.5) | |
| Urban | 56 (23) | 28 (50) | 28 (50) | |
| Marital status | 0.028 | |||
| Unmarried | 114 (46.9) | 51 (44.7) | 63 (55.3) | |
| Married | 116 (47.7) | 67 (57.8) | 49 (42.2) | |
| Widow | 13 (5.3) | 10 (76.9) | 3 (32.1) | |
| Smoking | 0.157 | |||
| Non-smokers | 212 (87.2) | 108 (50.9) | 104 (49.1) | |
| Active+ex-smokers | 31 (12.7) | 20 (64.5) | 11 (35.5) | |
| Co-morbidity | 0.837 | |||
| No | 208 (85.6) | 109 (52.4) | 99 (47.6) | |
| Yes | 35 (14.4) | 19 (54.3) | 16 (45.7) | |
| Close contact TB status | 0.164 | |||
| No TB | 175 (72) | 98 (56) | 77 (44) | |
| Drug susceptible TB | 36 (14.8) | 14 (38.9) | 22 (61.1) | |
| Drug resistant TB | 32 (13.2) | 16 (50) | 16 (50) | |
| Baseline weight (kg) | 0.229 | |||
| ≤40 | 92 (37.9) | 53 (57.6) | 39 (42.4) | |
| >40 | 151 (62.1) | 75 (49.7) | 76 (50.3) | |
| Baseline chest X-ray | 0.398 | |||
| No cavitations | 96 (39.5) | 73 (50.7) | 71 (49.3) | |
| Cavitations | 144 (59.3) | 54 (56.2) | 42 (43.8) | |
| Registration category | 0.002 | |||
| New | 21 (8.6) | 5 (23.8) | 16 (76.2) | |
| Relapse | 33 (13.6) | 21 (63.6) | 12 (36.4) | |
| Treatment failure | 183 (67.1) | 82 (50.3) | 81 (49.7) | |
| Default | 3 (1.2) | 3 (100) | 0 (0) | |
| Other | 23 (9.5) | 17 (73.9) | 6 (26.1) | |
| Previous TB treatment center | 0.002 | |||
| Public | 133 (54.7) | 64 (48.1) | 69 (51.9) | |
| Private | 68 (28) | 44 (64.7) | 24 (35.3) | |
| Public–private mix | 21 (8.6) | 15 (71.4) | 6 (28.6) | |
| No history of TB treatment | 21 (8.6) | 5 (23.8) | 16 (76.2) | |
| History of SLD use | 1.112 | |||
| No | 231 (95.1) | 119 (51.5) | 112 (48.5) | |
| Yes | 12 (4.9) | 9 (75) | 3 (25) | |
| Smear grading at baseline | 0.199 | |||
| Negative | 16 (6.6) | 5 (31.2) | 11 (68.6) | |
| Scantya, +1b | 14 (18.1) | 24 (54.5) | 20 (45.5) | |
| +2c, +3d | 180 (74.1) | 98 (54.4) | 82 (45.6) | |
| Site of TB | 0.515* | |||
| Pulmonary | 238 (97.9) | 125 (52.5) | 113 (47.5) | |
| Extra-pulmonary | 3 (1.2) | 1 (33.3) | 2 (66.7) | |
| Pulmonary+extra-pulmonary | 2 (0.8) | 2 (100) | 0 (0) | |
A high degree of drug resistance (median five drugs, range 2–8) was observed. Among three first-line anti-TB drugs (PZA, E, and S), the rate of resistance was highest for PZA (97.5%) followed by E (81.1%) and S (70%). High proportion of patients was resistant to all five first-line anti-TB drugs (62.6%) and SLD (55.1%). Resistance to Ofx was observed in 52.7% patients. Sputum isolate from four patients (1.6%) was resistant to two drugs (HR), 18 (7.4%) to three drugs (16 to HRZ, 1 to HRS, and 1 to HRE), 33 (13.6%) to four drugs (13 to HREZ, 12 to HRZS, 7 to HRZ+ofx, 1 to HRE+Ofx), 96 (39.5%) to five drugs (62 to HREZS, 28 to HREZ+Ofx, 5 to HRZS+Ofx, 1 to HREZ+Eto), 83 (34.2%) to six drugs (78 to HREZS+ofx, 2 to HREZS+Eto, 1 HREZS+Cm, 1 to HREZ+Ofx+Eto, 1 to HRZ+Am+Km+Cm), eight to (3.3%) to seven drugs (HREZS+Ofx+Eto) and one to eight drugs (HREZS+Am+Km+Cm) (Table 2).
Anti-tuberculosis drug susceptibility patterns.
| Variables | No. (%) |
|---|---|
| Drugs resistance at baseline visit | |
| 2–4 drugs | 55 (22.6) |
| 5–6 drugs | 179 (73.7) |
| >6 drugs | 9 (3.7) |
| Resistance to pyrazinamide | 236 (97.1) |
| Resistance to ethambutol | 197 (81.1) |
| Resistance to streptomycin | 170 (70) |
| Resistance to all five first line drugs | 153 (62.6) |
| Resistance to second line drugs | 134 (55.1) |
| Resistance to ofloxacin | 128 (52.7) |
| Resistance to ethionamide | 13 (6.6) |
| Resistance to capreomycin | 3 (1.2) |
| Resistance to amikacin | 2 (0.8) |
| Resistance to kenamcyin | 2 (0.8) |
In the univariate analysis, patient marital status, history of TB treatment, registration category, and center(s) that had provided previous TB treatment were significantly associated with Ofx resistance. In multivariate analysis, Ofx resistance had statistically significant positive association with previous TB treatment at private (OR=1.953, p=0.034) and public private mix (PPM) (OR=2.824, p=0.046) sectors (Table 3).
Predictors of ofloxacin resistance.
| Variables | Ofloxacin resistance | Univariate analysis | p-Value | Multivariate analysis | p-Value |
|---|---|---|---|---|---|
| No (%) | OR (95%CI) | OR (95%CI) | |||
| Gender | |||||
| Female | 135 (55.6) | Referent | |||
| Male | 108 (44.4) | 1.317 (0.792–2.190) | 0.288 | ||
| Age (years) | |||||
| 10–20 | 71 (29.2) | Referent | |||
| 21–40 | 112 (46.1) | 1.008 (0.556–1.826) | 0.980 | ||
| 41–60 | 48 (19.8) | 1.149 (0.551–2.394) | 0.711 | ||
| >60 | 12 (4.9) | 2.917 (0.729–11.675) | 0.130 | ||
| Nationality | |||||
| Pakistani | 223 (91.8) | Referent | |||
| Afghan | 20 (8.2) | 1.107 (0.442–2.777) | 0.828 | ||
| Residence | |||||
| Rural | 187 (77) | Referent | |||
| Urban | 56 (23) | 0.870 (0.479–1.581) | 0.648 | ||
| Marital status | |||||
| Unmarried | 114 (46.9) | Referent | Referent | ||
| Married | 116 (47.7) | 1.689 (1.003–2.846) | 0.049 | 1.589 (0.921–2.741) | 0.096 |
| Widow | 13 (5.3) | 4.118 (1.076–15.757) | 0.039 | 3.536 (0.903–13.848) | 0.070 |
| Smoking | |||||
| Non smokers | 212 (87.2) | Referent | Referent | ||
| Active+ex-smokers | 31 (12.7) | 1.751 (0.800–3.833) | 0.161 | 1.649 (0.727–3.739) | 0.231 |
| Co-morbidity | |||||
| No | 208 (85.6) | Referent | |||
| Yes | 35 (14.4) | 1.079 (0.526–2.213) | 0.837 | ||
| Close contact TB status | |||||
| No TB | 175 (72) | Referent | Referent | ||
| Drug susceptible TB | 36 (14.8) | 0.500 (0.240–1.041) | 0.064 | 0.686 (0.288–1.637) | 0.396 |
| Drug resistant TB | 32 (13.2) | 0.786 (0.369–1.671) | 0.531 | 0.832 (0.371–1.863) | 0.654 |
| Baseline weight (kg) | |||||
| ≤40 | 92 (37.9) | Referent | |||
| >40 | 151 (62.1) | 0.726 (0.431–1.224) | 0.230 | ||
| Baseline chest X-ray | |||||
| No cavitations | 96 (39.5) | Referent | |||
| Cavitations | 144 (59.3) | 1.250 (0.744–2.101) | 0.399 | ||
| Registration category | |||||
| New | 21 (8.6) | Referent | Referent | ||
| Relapse | 33 (13.6) | 5.600 (1.638–19.147) | 0.006 | 0.550 (1.046–2.608) | 0.376 |
| Treatment failure | 183 (67.1) | 3.240 (1.134–9.258) | 0.028 | 0.339 (0.129–1.237) | 0.112 |
| Other | 26 (10.7) | 10.667 (2.747–41.423) | 0.001 | ||
| Previous TB treatment center | |||||
| Public | 133 (54.7) | Referent | 0.027 | Referent | 0.034 |
| Private | 68 (28) | 1.197 (1.082–3.611) | 0.053 | 1.953 (1.053–3.624) | 0.046 |
| Public–private mix | 21 (8.6) | 2.695 (0.986–7.331) | 0.044 | 2.824 (1.021–7.182) | 0.075 |
| No history of TB treatment | 21 (8.6) | 0.337 (0.117–0.973) | 0.378 (0.130–1.104) | ||
| History of SLD use | |||||
| No | 231 (95.1) | Referent | Referent | ||
| Yes | 12 (4.9) | 2.824 (0.745–10.996) | 0.127 | 2.164 (0.542–8.642) | 0.275 |
| Smear grading at baseline | |||||
| Negative | 5 (31.2) | Referent | 0.117 | Referent | |
| Scantya, +1b | 24 (54.5) | 0.785–8.874 | 0.084 | 2.276 (0.641–8.082) | 0.203 |
| +2c, +3d | 98 (54.4) | 0.878–7.876 | 2.137 (0.675–6.766) | 0.197 | |
The present study revealed a high degree of drug resistance. The majority of MDR-TB patients (62.6%) were resistant to all five first line anti-TB drugs, and more than half (55.1%) were resistant to SLD. FQ resistance in MDR-TB patients observed in present study (52.7%) was much higher than that reported in the Global Tuberculosis Report 2014 (17%),6 but is in line with reported increase of FQ resistance in MDR-TB patients in Pakistan (17.4% in 2005, 42.9% in 2009, and 53.9% in 2014).7,8 High rate of FQ resistance observed in current study, and its gradual increase in drug resistant TB in the country makes it an alarming issue. This could be attributed to many factors. Patients’ prior exposure to FQ has been cited as one reason for FQ resistant Mycobacterium tuberculosis (MTB) strains.11,12 A recent meta-analysis concluded that there is threefold greater risk of FQ resistance among TB patients who are exposed to FQ before diagnosis of TB.13 Reports from Pakistan and other developing countries attribute to frequent self medication of chest symptomatics in the community before they are actually diagnosed with TB and put on appropriate treatment.14–17 A median of more than 12 weeks delay between onset of TB symptoms and presentation at a TB treatment center has been reported by studies conducted in Pakistan.16,17 Because of easy access and low or no consultation fee, most poor patients in Pakistan with inadequate awareness about TB initially prefer local paramedics for seeking treatment for respiratory tract infections.18 On the other hand, patients having the financial means, due to easy access, short waiting periods, care givers better attitudes, and stereotyping private health sector as a provider of high quality care prefer private practitioners.16,17 Due to lack of diagnostic facilities and inadequate knowledge,19,20 paramedics and private practitioners often prescribe pharmacy driven broad spectrum FQ21 before referring patients to TB treatment centers to be treated. This shopping around of patients, and exposure to FQ before TB diagnosis is one of the most likely reason for high FQ resistance in TB patients in Pakistan. Non-prescription sale of FQ,22 its widespread use by general practitioners for lower respiratory tract infections other than TB,7,23 and availability of counterfeit and substandard preparations in developing countries24,25 are other possible reasons for high FQ resistance in TB patients in these countries. Although in the present study very few patients had the documented record of previous use of FQ, but due to its easy availability,22 and frequent prescription by clinicians for respiratory tract infections in the country, previous use of FQ in these patients cannot be ruled out. Failure to keep the minimum essential records of TB patients particularly in private health sector,19 and over-the-counter availability of FQ in the country22 make it difficult to trace history of previous use of FQ in TB patients.
In current study multivariate analysis showed previous TB treatment at private sector as a risk factor for FQ resistance in MDR-TB patients. Prescription of FQ to patients when TB is misdiagnosed with other lower respiratory tract infections, and inappropriate TB treatment by inadequately trained professionals in private sector could explain positive association between previous TB treatment at a private center and FQ resistance. Insufficient knowledge of private practitioners, deviation from TB management guidelines, inappropriate regimens, lack of supervision on treatment adherence, and no action on patient default has widely been reported in Pakistan.19,20,26 A study conducted at Lahore and Rawalpindi reported that only one out of 245 private practitioners correctly identified cough for >3 weeks alone as the main symptom suggesting pulmonary TB. Among them, 97% were unaware of TB treatment categories; all of them (100%) were unaware of guideline recommended treatment regimen, phases and duration; and none (0%) was observing DOTS strategy.19 Similarly poor practices by private practitioners have been reported in India:27,28 only six out of 106 private practitioners wrote a correct prescription with the correct regimen for TB patients. Over one third of them prescribed a single SLD which was an FQ in 70% cases.27 Similarly, 105 private practitioners were reported to prescribe 79 different regimens for treating TB containing very few guidelines compliant regimens.28 In addition, the huge and unregulated private sector of sub-standard anti-TB drugs (including FQ) is another contributing factor in development of FQ resistance.24,25 A study conducted in 10 TB high burden countries (including Pakistan) found that at least one third of all private sector dosages of first-line TB drugs fell outside of national and international treatment recommendations.25
In the present study, previous TB treatment at PPM sector also emerged as risk factor for FQ resistance. In Pakistan, the PPM strategy for TB management was initiated in 2006; and at present it engages 2300 private health care providers. The majority of PPM centers in the country involve for-profit-qualified private practitioners, where drugs are given free of cost to patients they are but charged for consultation. Very few studies have evaluated the effectiveness of PPM regarding case detection rate and TB treatment outcomes in Pakistan.18,29,30 A study conducted at a PPM center in Karachi has reported national figures comparable treatment success rate (86.3%), but a high default rate (8.7%) in TB patients.30 Similar TB treatment success rate of 87% in patients treated at PPM sector has been reported by another study conducted in the same city.18 Despite training, frequent interaction, and authors’ regular visits to study sites, in both studies only half of the practitioners enrolled remained active and committed.18,30 Due to scarcity of publication on practitioners’ knowledge and adherence to national guidelines for TB management at PPM in the country, it is difficult to explain the association between previous TB treatment at PPM centers and FQ resistance. The inertia of previous practice, charging fee for every visit, lack of proper supervision of treatment adherence, and practitioners’ low level of commitment at PPM sector18,30 may have resulted in inappropriate and irregular TB treatment and, in turn, FQ resistance. However this finding needs further investigation.
In current study due to high prevalence of FQ resistance, predominant TB type was Pre-XDR-TB (MDR-TB+resistance to FQ). This brought the suitability of the guidelines recommended standardized regimen (Am/Km-Lfx-Eto-Cs-Z) in patients in to question.31 Initiating therapy with Am/Km-Lfx-Eto-Cs-Z will result in suboptimal regimen (<4 active or likely active SLD) in the majority of patients until the availability of DST results, which by conventional method usually takes 6–8 weeks. This delay in initiating optimal regimen may result in poor treatment outcomes, amplification of further resistance, conversion of pre-XDR to XDR-TB, and its transmission in community. In the light of findings from the present previously conducted large studies,7,8 we suggest that the National Tuberculosis Control Program should evaluate MDR-TB patients’ countrywide data for FQ resistance. If the present findings are confirmed, then WHO updated recommended regimen (Am/Cm-Lfx-Eto-Cs-PAS-Z) for initiating treatment in MDR-TB patients in settings of high SLD resistance32 should be adopted.
Although at the time of study initiation PMDT unit LRH was the only center in Khyber Pukhtoonkhwa (one of the four provinces of Pakistan) where MDR-TB was treated, but being study from a single center, and limited sample size, its results cannot be generalized. A multicenter study with larger sample size is suggested to confirm the present findings.
ConclusionConsidering the burden of MDR-TB in Pakistan and the importance of FQs in treating drug-resistant TB, the high prevalence of FQ resistant MDR-TB patients observed in this study is alarming. The adoption of more restrictive policies to control non-prescription sale of FQ, and its rational use by physicians, is urgently needed to prevent further increase in FQ resistant MTB strains. In order to stop the further escalation of FQ resistance in Pakistan, we recommend that the country should increase its efforts to increase public awareness about TB and the hazards of self-medication with antibiotics; train registered paramedics and practitioners, particularly in the private and PPM sectors; and to develop people trust on public health sector by making it more extensive, appealing, and flexible.
Conflicts of interestThe authors declare no conflicts of interest.
The authors acknowledge all patients and staff of study site for their support to conduct this study.