With the advances in surgical treatment, antibiotic therapy and the current resources for accurate diagnosis and differentiated approaches to each type of osteomyelitis, better results are being obtained in the treatment of this disease. After a careful literature review carried out by a multiprofessional team, some conclusions were made in order to guide medical approach to different types of osteomyelitis, aiming to obtain better clinical outcomes and reducing the social costs of this disease. Acute and chronic osteomyelitis are discussed, with presentation of the general epidemiological concepts and the commonly used classification systems. The main guidelines for the clinical, laboratory and imaging diagnosis of infections are discussed, as well as the guidelines for surgical and antimicrobial treatments, and the role of hyperbaric oxygen as adjuvant therapy.
With the advances in surgical treatment, antibiotic therapy, and the current resources for accurate diagnosis and differentiated approaches to each type of osteomyelitis, better results are being obtained in the treatment of this disease. On the other hand, as a result of high-energy trauma with extensive damage to soft tissues requiring more aggressive treatments for open and closed fractures, we have seen a higher number of infections arising from surgical procedures related to these traumatic lesions, which often take the form of post-traumatic osteomyelitis and serious soft-tissue infections. In this scenario, with the progressive increase in traumatic injuries and their associated complications, osteomyelitis – particularly post-traumatic osteomyelitis – is a significant public health problem. The objective of this review article is to indicate some recommendations based on scientific evidence that will guide the medical approach to different types of osteomyelitis, aiming to obtain better clinical outcomes and at reducing the social costs of this disease. Acute and chronic osteomyelitis are discussed, with presentation of the general epidemiological concepts and the commonly used classification systems. The main guidelines for clinical, laboratory and imaging diagnosis of infections are discussed, as well as the guidelines for surgical and antimicrobial treatments, and the role of hyperbaric oxygen as adjuvant therapy.1 The conclusions of this multidisciplinary review are summarized below.
- I.
Which classification should be used?
- 1.
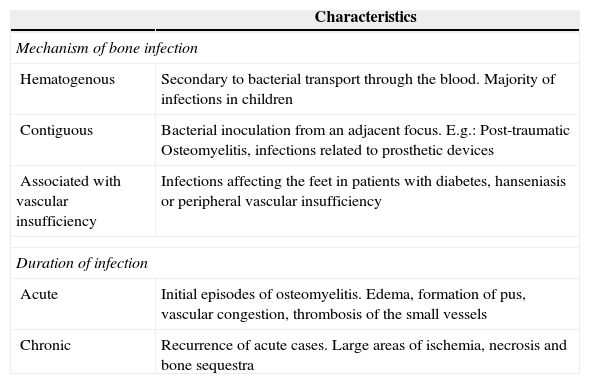
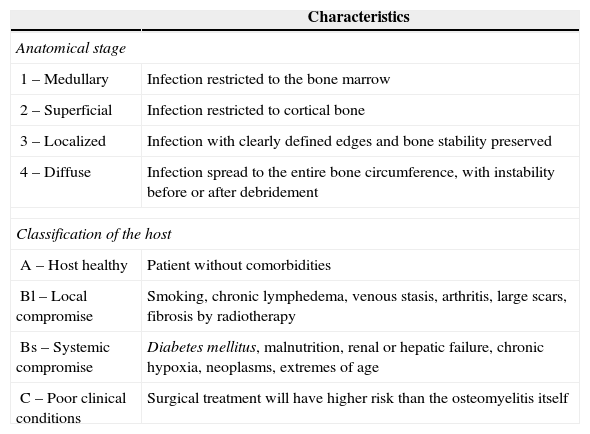
An ideal classification of osteomyelitis should consider the different aspects that influence its pathophysiology, addressing all the possible etiologies and parameters of temporal evolution. It should also be closely correlated with the histological data and should include proposals for the treatment of each classification stage. In general, the Waldvogel classification2 is recommended for its greater clinical applicability, and the Cierny and Mader classification3 for its clearly defined surgical treatment proposals (Tables 1 and 2).
Table 1.Waldvogel classification of osteomyelitis.
Characteristics Mechanism of bone infection Hematogenous Secondary to bacterial transport through the blood. Majority of infections in children Contiguous Bacterial inoculation from an adjacent focus. E.g.: Post-traumatic Osteomyelitis, infections related to prosthetic devices Associated with vascular insufficiency Infections affecting the feet in patients with diabetes, hanseniasis or peripheral vascular insufficiency Duration of infection Acute Initial episodes of osteomyelitis. Edema, formation of pus, vascular congestion, thrombosis of the small vessels Chronic Recurrence of acute cases. Large areas of ischemia, necrosis and bone sequestra Adapted from Ref. 2.Table 2.Cierny and Mader classification of osteomyelitis.
Characteristics Anatomical stage 1 – Medullary Infection restricted to the bone marrow 2 – Superficial Infection restricted to cortical bone 3 – Localized Infection with clearly defined edges and bone stability preserved 4 – Diffuse Infection spread to the entire bone circumference, with instability before or after debridement Classification of the host A – Host healthy Patient without comorbidities Bl – Local compromise Smoking, chronic lymphedema, venous stasis, arthritis, large scars, fibrosis by radiotherapy Bs – Systemic compromise Diabetes mellitus, malnutrition, renal or hepatic failure, chronic hypoxia, neoplasms, extremes of age C – Poor clinical conditions Surgical treatment will have higher risk than the osteomyelitis itself Adapted from Ref. 3.
- 1.
- II.
Which subsidiary tests are important for the diagnosis of osteomyelitis?
- 2.
The diagnosis of osteomyelitis considers a range of clinical signs and symptoms, laboratory tests, imaging studies and histological analyses, as well as the identification of pathogens by means of bone tissue or blood cultures.
- 3.
In terms of laboratory tests, serum leukocyte count and inflammatory markers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), can assist in the initial diagnosis of osteomyelitis. However, these are non-specific tests and are more useful in the control of treatment.
- 4.
The histology of biological samples should be carried out in all suspect cases, and bone biopsy, soft tissue, and bone sequestra can confirm the diagnosis of osteomyelitis.
- 5.
A definitive diagnosis of osteomyelitis is obtained with microbiological identification of the pathogen in bone, through a bone biopsy. Samples obtained through swabs of the fistula or secretions for use in cultures will result in false positive results, as they identify microorganisms that colonize the skin. At least three different samples of bone tissue should be obtained, in order to increase the positivity of the test. Antimicrobial therapy should be started after collecting culture samples or at the same time as anesthetic induction. Patients should stop any antibiotics two weeks before collecting culture samples, if possible. In cases of osteomyelitis with osteosynthesis or in infected arthroplasties, sonication of the implants significantly increases the identification of pathogens.
- 6.
The use of complementary imaging methods can be important in the early diagnosis of osteomyelitis. It can also assist in rapid start of treatment and follow-up, enabling ineffective treatments to be modified. In acute osteomyelitis, a plain radiography shows osteomyelitis only after two weeks. Magnetic resonance imaging (RMI) is considered the main type of imaging in the evaluation of bone infections, as it can detect osteomyelitis as early as three to five days of infection. Computed tomography (CT) is of little use in the diagnosis of acute infection, but is important for investigating bone sequestra and planning surgery. Three-phase bone scintigraphy, scintigraphy with Gallium-67 and the positron emission tomography (PET-CT) are examinations that help in the differentiation of doubtful cases.
- 2.
- III.
What are the recommendations for the treatment of osteomyelitis?
- 7.
The success of osteomyelitis treatment, particularly in cases related to implants, is closely linked to extensive surgical debridement and adequate antibiotic therapy.
- 8.
Starting empirical antibiotics in anesthetic induction prevents the risks of bacteremia arising from surgical manipulation of infection without adequate antibiotic coverage. Yet, it does not interfere with the positivity of cultures taken during the procedure. Empirical antibiotic can also be started after collecting culture samples in non-septic patients.
- 9.
Empirical coverage of Staphylococcus aureus is recommended, given the epidemiological importance of this agent. The local prevalence of methicillin resistance, even in community-acquired cases, is variable and should also be observed.
- 10.
Acute infections can be treated initially with extensive surgical cleaning associated with antibiotic therapy lasting four to six weeks. Chronic infections should be treated with extensive surgical debridement, removal of any implants and antibiotic therapy lasting three to six months.
- 7.
Osteomyelitis, which was named by Nelaton in 1844, is one of the oldest reported diseases known to the scientific community. However, the available epidemiological data are scarce, probably due to the different pathophysiological mechanisms involved in the genesis of the disease, which makes it difficult to estimate the incidence and prevalence in the general population.4,5
Osteomyelitis can be defined as an inflammation of the bone tissue caused by an infectious agent. This infection may be hematogenic, contiguous to an adjacent infectious focus, or even the result of direct bacterial inoculation from a traumatic mechanism. In general, hematogenous osteomyelitis is caused by a single agent, while other types can show polymicrobial infection.6,7 Hematogenous osteomyelitis has more consolidated data in the medical literature, and is considered a predominantly pediatric disease, with 85% of patients aged below 17 years.8 In adult patients, it is estimated that 47–50% of all osteomyelitis are post-traumatic. Vertebral osteomyelitis occurs in 2–7% of patients.4,9
Chronic osteomyelitis represents a major health problem due to its significant morbidity and low mortality rate.3,5,8,10 This infection occurs in approximately 5–50% of open fractures, in less than 1% of closed fractures with osteosynthesis, and in 5% of acute hematogenous disease.5 The main problem associated with chronic bone infection is the capacity of the microorganisms to remain in necrotic bone tissue for long periods, especially in tissues that has not undergone adequate surgical debridement.
Classification systems for osteomyelitisOsteomyelitis is a highly heterogeneous disease in its clinical presentation, pathophysiology and treatment. The various clinical syndromes that comprise this entity, although grouped under the same name, should be classified according to their common characteristics, enabling standardization of conducts and comparison of the outcomes of different clinical studies.11
Various classification systems have been described in the medical literature, and the adoption of any one should be suitable for the particularities of each treatment center. Recently, new classifications have been described.12 However, further clinical studies are needed before they can be adopted. In general, the Waldvogel classification2 is recommended for its greater clinical applicability, and the Cierny and Mader3 classification for its clearly defined treatment proposals.
Waldvogel classificationThis classification was described in 1970 and is still the most important and widely used system in clinical studies. The authors divide osteomyelitis according to its physiopathology and the duration of infection. Based on the physiopathology, infections are classified into three groups: hematogenous osteomyelitis; osteomyelitis secondary to a contiguous focus of infection; and osteomyelitis associated with peripheral vascular insufficiency (Table 1). Based on the length of evolution, the infections are classified as acute osteomyelitis and chronic osteomyelitis (recurrences). The authors do not determine a time of evolution that would distinguish between chronic and acute cases.
Cierny and Mader classificationThe Cierny and Mader classification was described in 1984, as an attempt to address some aspects that were not covered by previous classifications. In this classification, osteomyelitis is divided according to bone anatomy and physiological factors of the host (Table 2). The authors describe four anatomical stages, according to the bone involvement, and three types of host, depending on the patient's clinical conditions. It was developed mainly for infections in long bones.
DiagnosisCorrect diagnosis of bone infections presents many difficulties, as many tests are not widely standardized. The clinical signs and symptoms, along with the inflammatory markers, are also nonspecific. Imaging examinations may elucidate very little in the acute phase of the disease and may not be very specific in the chronic phase, and obtaining tissue samples for culture does not always help confirming the diagnosis. Diagnosis of osteomyelitis requires a set of clinical signs and symptoms, laboratory tests, imaging studies, histological analysis and, finally, the identification of pathogens by means of bone tissue or blood cultures, particularly in cases of hematogenous osteomyelitis.13
Clinical suspicion is critical to start medical investigation, and its manifestations depend on several factors, such as the length of infection (acute or chronic), infection site and type of bone involved.13,14
In acute forms of osteomyelitis and in those of hematogenous origin, local symptoms, such as pain, heat, edema, and hyperemia, and systemic symptoms, such as fever, general malaise, and adynamia, appear up to two weeks after the initial infection. However, the clinical presentation of the disease can be quite variable. Diagnosis is easier in patients who present cutaneous fistula or open wound with bone exposure following open fractures, but very difficult in patients who have only progressive pain.14 In chronic forms of osteomyelitis, the clinical presentation is highly variable. The systemic symptoms are usually absent and the local symptoms, such as hyperemia, heat, edema and fistulization, often appear intermittently, or even years after the beginning of bone infection.14
Laboratory testsAcute infections are often associated with leukocytosis and neutrophilia – a change that is rarely found in chronic osteomyelitis. Inflammatory markers, such as ESR and CRP, are often elevated in acute hematogenous osteomyelitis in children. However, these are nonspecific tests and are more important in the control of treatment.15,16 The serum procalcitonin levels for the diagnosis or follow-up of hematogenous osteomyelitis in children or in diabetic patients did not prove effective in several studies.17–19 Serum level of interleukin-6 is most commonly studied as a diagnostic tool of bone infections associated with joint prosthesis.20
Histological testsSamples of bone, soft tissue and bone sequestra should be sent for histological analysis after biopsy or surgical debridement, as these can confirm the diagnosis of osteomyelitis.14,21,22 In acute osteomyelitis, polymorphonuclear leukocytes are predominant, while in chronic forms, lymphocytes, osteoblasts and osteoclasts are predominant.21 In suspicious cases of osteomyelitis, histological examination may lead to diagnostic confirmation in up to 50% of patients.23 Frozen samples of bone tissues obtained during surgery with more than five neutrophils per field present sensitivity ranging from 43% to 84% and specificity of 93–97% in bone infections associated with orthopedic implants.14,24
Microbiological testsAt least three bone samples should be obtained, in order to increase the positivity rate of the test. Antimicrobial therapy should be started after collecting culture samples or at the same time as anesthetic induction.22,23 Patients should stop any antibiotics two weeks before collecting culture samples, if possible. Slow-growing bacteria, such as Propionibacterium acnes, may be associated with osteomyelitis with osteosynthesis, and in these cases it is important to prolong the incubation time of the culture plates for up to 14 days.25 In fact, bone cultures can produce false-negative results in up to 40% of cases, especially in patients using antibiotics.26
Sonication significantly increases the identification of the pathogens when osteomyelitis occurs in the presence of osteosynthesis, including infected arthroplasties. Sonication consists of subjecting the implants to low-frequency ultrasound, and consequent rupture of protective extracellular polymeric surface over the bacteria contained in the biofilms. The bacteria, thus released from the biofilms into the liquid medium, remain viable and are cultivated in solid and liquid culture media.27
Imaging diagnosis and nuclear medicineIn acute osteomyelitis, initial plain radiography does not show any changes. After around three to four days there may be an increase in soft tissues. Bone changes appear after two weeks, and poorly delineated lytic lesions can also be observed, simulating an aggressive lesion. A lamellar periosteal reaction is also evident. Plain radiographies have a positivity rate of only 20% after two weeks, but are necessary to rule out other orthopedic illnesses (tumors, fractures).28,29
MRI is considered the main type of imaging in the evaluation of bone infections, revealing changes as early as the first few days of the disease. Bone marrow edema is also evident in MRI (as poorly defined areas of hyposignal in T1-weighted sequences and hypersignal in T2, with post-contrast enhancement). As the disease progresses, abscesses appear, with typical peripheral enhancement in the contrast phase. In children, the infection characteristically crosses the growth cartilage, unlike neoplastic changes. The specificity of MRI is higher than that of bone scintigraphy in the diagnosis of infection.30–32
CT is of little utility in the diagnosis of acute infection. Its role is restricted to the study of bone sequestra in case of subacute and chronic infections, indicating potential infection activity.33,34
Ultrasound examination may be of use, especially in younger patients, as it reveals edema of the soft tissues around the bone, periosteal thickening, and subperiosteal collections. An area of hyperemia can also be observed in the color Doppler. This method provides very little data on intra-osseous extension, and is of limited use in this regard.30,35
Imaging methods are of little utility in the therapeutic management of bone infections. Radiographic changes may still be present, despite adequate treatment. In these cases, functional methods, especially PET-CT, play a more important role.33,34,36
Nuclear medicine uses radiotracers with known biological properties in order to outline an image of a physiological process of the organism. Some of the most common indications of nuclear medicine methods are in cases of suspected osteomyelitis with doubtful clinical or radiographic signs, when there are image artifacts in the radiological methods and in the developmental follow-up or response to treatment.29,37,38
PET-CT is a technique that uses positron-emitting isotopes to form images, the main one being fluorine-18-labeled fluorodeoxyglucose. It provides improved spatial resolution, better sensitivity, and better specificity when compared to conventional scintigraphy (96% and 91%, respectively). It can be considered one of the best techniques in nuclear medicine, but it is a high-cost examination and is only available in a few diagnostic centers, which limits its use.
Bone scintigraphy is an examination that has historically been used to differentiate osteomyelitis from soft tissue infections. It uses diphosphonate radiotracers marked with technetium-99 metastable isotope (99mTc), methylene diphosphonate (99mTc-MDP) being one of the most commonly used. It is performed in the so-called three-phase mode: the first flow phase with dynamic images acquired immediately after intravenous injection of the radiotracer, for 1min; the second phase, steady-state, with static images of the region of greatest interest, acquired 5min after injection of the radiotracer; and the third phase, the late phase, with whole-body images, acquired after 2h of injection of the radiotracer. It presents reasonable sensitivity (70–89%), but low specificity (16–36%).32–34
Gallium scintigraphy uses gallium-67 citrate, an iron analog radiotracer that concentrates in inflamed tissues due to the higher blood flow and increased concentration of transferrin, to which it binds. It should be used in conjunction with bone scintigraphy for the evaluation of cases of osteomyelitis, where it shows a greater uptake of the radiotrace and infers the presence of active infectious process.39
Indium-111 marked leukocyte scintigraphy is considered the best method of nuclear medicine for assessing patients with osteomyelitis, because it is independent of bone remodeling. Because it is a high-cost procedure, and complex to implement, it is available in very few diagnostic centers. It shows good sensitivity (84%) and specificity (80%).28
Antimicrobial treatmentThe rate and extent of antibiotic penetration in bone tissues are seen as determining factors for therapeutic success in osteomyelitis.40 On the other hand, penetration of an antibiotic into infected bone tissue depends on its pharmacological characteristics, the degree of vascularization, good conditions of soft tissues, and the presence of foreign bodies.41 Integrating information related to tissue concentration in clinical practice is a stumbling block in the process of antimicrobial selection for the treatment of bone infections.
Antibiotics with a high bone/serum concentration ratioThe decision on the clinical usefulness of an antibiotic in osteomyelitis should combine studies on bone concentration with the results of clinical studies in patients with osteomyelitis.40,42
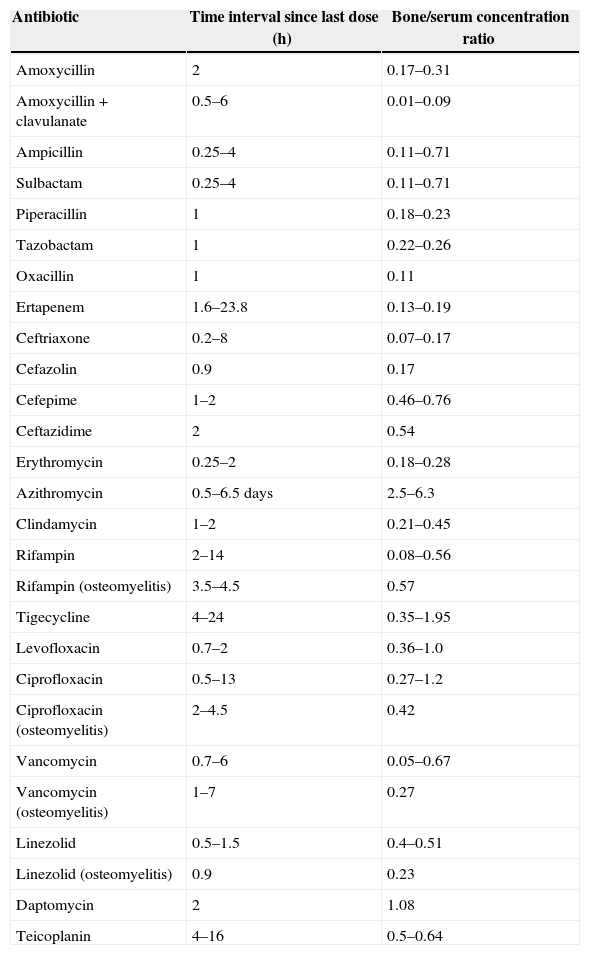
The majority of bone penetration studies are performed in patients undergoing hip replacement surgery, and samples obtained are from uninfected bones. With this in mind, Table 340,42 shows the bone concentration of the antibiotics presented in clinical studies not involving humans.
Bone penetration of antibiotics.
| Antibiotic | Time interval since last dose (h) | Bone/serum concentration ratio |
|---|---|---|
| Amoxycillin | 2 | 0.17–0.31 |
| Amoxycillin+clavulanate | 0.5–6 | 0.01–0.09 |
| Ampicillin | 0.25–4 | 0.11–0.71 |
| Sulbactam | 0.25–4 | 0.11–0.71 |
| Piperacillin | 1 | 0.18–0.23 |
| Tazobactam | 1 | 0.22–0.26 |
| Oxacillin | 1 | 0.11 |
| Ertapenem | 1.6–23.8 | 0.13–0.19 |
| Ceftriaxone | 0.2–8 | 0.07–0.17 |
| Cefazolin | 0.9 | 0.17 |
| Cefepime | 1–2 | 0.46–0.76 |
| Ceftazidime | 2 | 0.54 |
| Erythromycin | 0.25–2 | 0.18–0.28 |
| Azithromycin | 0.5–6.5 days | 2.5–6.3 |
| Clindamycin | 1–2 | 0.21–0.45 |
| Rifampin | 2–14 | 0.08–0.56 |
| Rifampin (osteomyelitis) | 3.5–4.5 | 0.57 |
| Tigecycline | 4–24 | 0.35–1.95 |
| Levofloxacin | 0.7–2 | 0.36–1.0 |
| Ciprofloxacin | 0.5–13 | 0.27–1.2 |
| Ciprofloxacin (osteomyelitis) | 2–4.5 | 0.42 |
| Vancomycin | 0.7–6 | 0.05–0.67 |
| Vancomycin (osteomyelitis) | 1–7 | 0.27 |
| Linezolid | 0.5–1.5 | 0.4–0.51 |
| Linezolid (osteomyelitis) | 0.9 | 0.23 |
| Daptomycin | 2 | 1.08 |
| Teicoplanin | 4–16 | 0.5–0.64 |
The success of osteomyelitis treatment, particularly in cases related to implants, depends on extensive surgical debridement and adequate and effective antibiotic therapy.43,44 Starting empirical antibiotics in anesthetic induction prevents the risks of bacteremia arising from surgical manipulation of infection without adequate antibiotic coverage. Yet, it does not interfere with the positivity of cultures taken during the procedure. Empirical antibiotic can also be started after collecting culture samples in non-septic patients.
The duration of antibiotic therapy varies from four weeks to six months, and the treatment should be adjusted based on the results of the cultures collected, where necessary.45,46 Acute infections can be treated initially with extensive surgical cleaning associated with antibiotic therapy lasting four to six weeks.
Chronic infections should be treated with extensive surgical debridement and removal of any synthesis materials, which can be replaced during the same surgical procedure if there is orthopedic indication. Due to biofilm formation, the total administration time of antibiotics in these infections is three to six months.47 See Table 4.
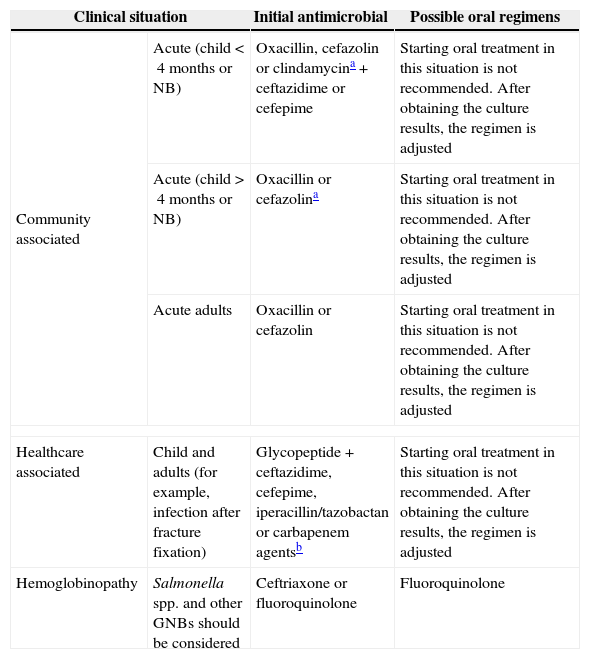
Suggested empirical initial antimicrobial regimens for osteomyelitis.
| Clinical situation | Initial antimicrobial | Possible oral regimens | |
|---|---|---|---|
| Community associated | Acute (child<4 months or NB) | Oxacillin, cefazolin or clindamycina+ceftazidime or cefepime | Starting oral treatment in this situation is not recommended. After obtaining the culture results, the regimen is adjusted |
| Acute (child>4 months or NB) | Oxacillin or cefazolina | Starting oral treatment in this situation is not recommended. After obtaining the culture results, the regimen is adjusted | |
| Acute adults | Oxacillin or cefazolin | Starting oral treatment in this situation is not recommended. After obtaining the culture results, the regimen is adjusted | |
| Healthcare associated | Child and adults (for example, infection after fracture fixation) | Glycopeptide+ceftazidime, cefepime, iperacillin/tazobactan or carbapenem agentsb | Starting oral treatment in this situation is not recommended. After obtaining the culture results, the regimen is adjusted |
| Hemoglobinopathy | Salmonella spp. and other GNBs should be considered | Ceftriaxone or fluoroquinolone | Fluoroquinolone |
There is no antimicrobial regimen that is perfect for every situation. The ability of rifampin in erradicating slow-growing bacteria in biofilms is well known. Thus, the suggestion to add rifampin to another drug with activity against S. aureus is recurrent in the literature, but this drug should never be used as monotherapy.48
Surgical treatmentHematogenous osteomyelitisIn order to optimize the surgical treatment of osteomyelitis, it is essential to stage the disease correctly. This includes investigating inflammatory activity and culture tests, and conducting imaging examinations.49–52 Sometimes infection in the pediatric age group can be confused with other oncological diseases that occur in this age group.53
Surgical treatment is mandatory when abscess is present. Surgical drainage associated with debridement is performed after confirmation of the diagnosis by bone biopsy in the operating room, with all the resources of asepsis and antisepsis.54
The surgical approach may be open surgery, arthroscopy or puncture/aspiration and flushing. The use of flushing under excessive pressure should be avoided, because in addition to causing injury to the soft parts and bone, the pressure can inoculate microorganisms deeply into the tissues.
Adequate debridement is the best predictor of success in the treatment of osteomyelitis. The surgical approach should be of the “oncology” type, i.e. with broad resection. Nowadays, a wide variety of surgical techniques are available for the reconstruction of both bone and soft tissues.49,52,54
Acute post-traumatic osteomyelitisThe treatment of acute osteomyelitis is surgical, particularly in the presence of an implant, because early bacterial identification and effective debridement are the only ways to save this implant. The surgeon should heed the clinical signs of a possible infection. During the postoperative period, when there are pain, local hyperemia, inflammation, serous exsudate and suspicion of a hematoma at the surgical site, the surgeon must act quickly, taking the patient back to the operating room for debridement and cultures.55
The most important factor for a successful treatment of patients with bone infection is the quality of debridement. The debridement must achieve a clean and viable wound through a non-traumatic exposure. In acute infection, surgical drainage and copious flushing of the cavity significantly reduce bacterial load at the site. Flushing should be performed with saline solution, with a total volume of 3–9L, and there is a direct relationship between the amount of saline solution used and the reduction of bacterial load.56–58
In situations in which there is a dead space after the removal of devitalized tissues, the use of polymethylmethacrylate cement impregnated with an antibiotic for local release is a good option. The high local concentration of antibiotics obtained using this technique is far above the MIC for the majority of microorganisms, and it would be impossible to achieve this concentration with the use of systemic antibiotics, due to associated toxicity. The antibiotics used in bone cement must not be thermolabile, due to the exothermic reaction of polymerization of polymethylmethacrylate, which inactivates these agents.59,60
Chronic osteomyelitisIn the approach to a patient with chronic osteomyelitis, the choice between palliative treatment and a curative approach should be considered. Surgery is currently the only form of cure in almost all cases; however, it is not always the best option. Therefore, a multidisciplinary approach is important in the assessment of each case, in order to decide on the best treatment.
The steps in the treatment of chronic osteomyelitis consist of correct microbiological diagnosis; improvement of the host's defenses; stabilization of underlying diseases; correct anatomical localization of bone involvement; adequate antimicrobial therapy; surgical debridement of all devitalized tissue; repair of soft tissues; and bone reconstruction and rehabilitation.6
All devitalized tissues need to be removed, and the surgical technique used will depend on the extent of the bone lesion.57,61–67 Wound closure by any means is imperative when vital structures (e.g., vessels, nerves, tendons, bone) are exposed, which may often require local flaps, or more complex flaps located further away (microsurgical). Only complete resection of all the devitalized tissues, with the establishment of adequate blood flow, will lead to effective systemic antimicrobial therapy and resolution of the infection. A resection margin of 5mm55 should be respected.
The use of antibiotic-coated cement may be an option in cases where there is dead space to be filled after the debridement and before the site is definitively closed. Commercially available antibiotic-impregnated cement spacers may be used for this purpose, but manual mixing of the antibiotic cement at the time of use is possible. The most commonly used antibiotic is vancomycin at a dosage of 2–4 grams per 40g of cement. Other antibiotics may also be used, provided they are not thermolabile, due to the exothermic reaction of the polymethylmethacrylate.59,60
Another measure is the use of vacuum-assisted closure, which has shown excellent results. Its correct use can significantly improve the condition of the soft tissue wound in terms of its granulation, characteristics of vascularization, and reducing its size.68–70
Adjuvant treatment – HBOHyperbaric oxygen therapy (HBO) is a form of adjuvant therapy that has been used worldwide for more than sixty years.71 It is used in patients with infectious, inflammatory, immunological, and ischemic tissue changes. The treatment involves respiration of 100% oxygen under hyperbaric conditions, i.e. under pressures artificially elevated above the atmospheric pressure at sea level, with the patient being placed inside a pressure-resistant hyperbaric chamber. In this setting, large quantities of oxygen under pressure penetrate the blood, are dissolved in the plasma, and reach the tissues. Tissue hyperoxygenation causes specific therapeutic effects, including stimulation of bacterial lysis by leukocytes, increase in proliferation of fibroblasts and collagen, and neovascularization of ischemic or irradiated tissues. The effects of HBO, such as immunomodulation,72 reduction in pro-inflammatory mediators, and reduction in effects of ischemia-reperfusion73 in ischemic tissues, are extremely useful for the treatment of infections. The use of hyperbaric oxygen (O2HB) is associated with all the other therapeutic measures, making them more effective. Wound healing time is accelerated, the esthetic results are better, and the final cost of treatment is also reduced.1
Conflicts of interestThe authors declare that the meeting for the elaboration of these guidelines was possible due to educational grants received from MSD, Bayer Schering-Pharma and Sanofi-Aventis. Any author received any fee.
Anibal Sosa – International Independent Consultant Services. Infectious Diseases and Clinical Microbiology, Boston, USA
Alejandro López – Universidade de Rosario, Bogotá, Colombia
Carlos Bergallo – Sanatorio Allende/Hospital Cordoba, Cordoba, Argentina
Carlos Julio Rodriguez Taveras – Hospital Central de Las Fuerzas Armadas, Santo Domingo, Distrito Nacional, Republica Dominicana
Carlos Ernesto Ferreira Starling – Hospital Vera Cruz, Belo Horizonte, Brazil
Carlos Kiffer – Universidade Federal de São Paulo, São Paulo, Brazil
Eliana Lima Bicudo dos Santos – Universidade de Brasília, Brasília, Distrito Federal, Brazil
Luis Elvin Molinedo Pérez – Universidade Maior de San Andrés, La Paz, Bolivia
George Barberio Coura Filho – Universidade de São Paulo, São Paulo, Brazil
Hernan Del Sel – Pontificia Universidade Católica de Buenos Aires/Hospital Britanico, Buenos Aires, Argentina
Hugo Eduardo Pezzarossi Zelaya – Infectocentro, Guatemala, Guatemala
Ivan Marinho – Hospital São Camilo Pompéia, São Paulo, Brazil
Jaime Luís Lopes Rocha – Universidade Federal do Paraná, Curitiba, Brazil
João Antonio Matheus Guimarães – Instituto Nacional de Ortopedia e Traumatologia, Rio de Janeiro, Brazil
Jorge Luiz Mello Sampaio – Universidade de São Paulo/Grupo Fleury, São Paulo, Brazil
José Luiz Amim Zabeu – Pontifícia Universidade Católica, Campinas, Brazil
Luiz Henrique Melo – Univille, Joinville, Brazil
Marcelo Abagge – Universidade Federal do Paraná, Curitiba, Brazil
Mauro José Costa Salles – Santa Casa de São Paulo, São Paulo, Brazil
Marcelo Simão Ferreira – Universidade Federal de Uberlândia, Uberlândia, Brazil
Marcelo Rosa de Rezende – Universidade de São Paulo, São Paulo, Brazil
Marcelo Bordalo Rodrigues – Universidade de São Paulo, São Paulo, Brazil
Osvandré Lech – Hospital-Escola São Vicente de Paulo, Passo Fundo, Brazil
Paulo Roberto dos Reis – Universidade de São Paulo, São Paulo, Brazil
Pilar Ramon-Pardo – Pan American Health Organization/World Health Organization, Washington, USA
Roberto Guarniero – Universidade de São Paulo, São Paulo, Brazil
Walter Ricioli Junior – Santa Casa de São Paulo, São Paulo, Brazil
The members of the Diretrizes Panamericanas para el Tratamiento de las Osteomielitis e Infecciones de Tejidos Blandos Group are listed in Appendix A.