D-dimer levels are significantly higher in COVID-19 patients with Pulmonary Thromboembolism (PTE) as compared to those without PTE, but its clinical utility is still uncertain.
PurposeTo determine the D-dimer performance for ruling out PTE in patients with COVID-19. We also assessed clinical and laboratory factors associated with the presence of PTE on CT Pulmonary Angiogram (CTPA).
MethodsRetrospective study involving all patients who presented at a tertiary care hospital from March 2020 to May 2021 with severe acute respiratory syndrome from COVID-19, who underwent CTPA and had D-dimer collected within 48 hours from CTPA. The D-dimer ability to classify patients with or without PTE according to CTPA was evaluated.
ResultsA total of 697 patients [382 (54.8%) men; mean (SD) age, 59 (20.5) years] were included, of which 71.5% required intensive care admission, 32.4% had PTE, and 35.6% died during hospitalization. PTE was independently associated with mortality [42.5% vs. 32.3%; p = 0.038]. D-dimer levels were higher in patients with PTE [9.1 (3.9; 20) vs. 2.3 (1.2; 5.1); p < 0.001]. Using the D-dimer cutoff of 0.5 μg/mL or above, sensitivity was 98.2% and specificity 5.7%. The 0.3 μg/mL threshold was associated with 100% of sensitivity for the presence of PTE, with which 99.1% of patients had increased values. ROC curve AUC was 0.77, demonstrating moderate discriminative power of D-dimers to detect PTE.
ConclusionsD-dimer levels are higher among COVID-19 hospitalized patients with PTE as compared to those without PTE and have moderate discriminative power to detect PTE, but its use to exclude PTE in this population may have limited clinical utility.
Since the outbreak of the Coronavirus Disease 2019 (COVID-19) pandemic, there have been over 400 million of SARS-CoV-2 infections worldwide, which resulted in more than five million deaths. COVID-19 is associated with hypercoagulability and increased risk of Venous Thromboembolism (VTE) events, which plays an important role in mortality from the disease.1 Studies have reported a Pulmonary Thromboembolism (PTE) incidence of 6.4%‒57%, with higher incidence rates among patients admitted to the intensive care unit.1–25
There is an association between the level of D-dimers and the incidence of thrombotic events in these patients, but elevated D-dimers are also often found in patients without thromboembolic events.1 As dyspnea and hypoxemia can be present in both COVID-19 pneumonia and PTE, this differentiation has become a major diagnostic challenge.
Computed Tomographic Pulmonary Angiography (CTPA) is the standard method of diagnostic imaging for pulmonary embolism due to its high negative and high positive predictive values, in addition to being able to evaluate alternative diagnoses. However, because of excessive radiation exposure, possible contrast reactions and costs, it still could be avoided when possible. It is well established that plasma D-dimer levels can be used for this purpose when in combination with clinical prediction scores of pretest probability, ruling out Pulmonary Embolism (PTE) and dismissing the need for CTPA in some cases.26–31
The role of D-dimers in the clinical decision rules for pulmonary embolism in patients with COVID-19 is still undetermined. The peculiarities of this population, that presents increased risk for thromboembolic events, but also commonly D-dimers elevation in its absence, justify the speculation that perhaps the previously used clinical decision rules for PTE diagnosis may not apply to this specific group. The abundant availability of D-dimer levels, routinely collected from patients hospitalized with COVID-19 for prognostic stratification, has provided data of uncertain clinical utility to health professionals in the last two years.
Thus, the aim of this study was to determine the D-dimer performance for ruling out PTE in patients with COVID-19. In addition, we sought to determine the incidence of PTE in COVID-19 patients, identifying its associations with clinical and laboratory parameters.
Material and methodsWe conducted a retrospective study involving all consecutive patients who presented at Hospital de Clinicas de Porto Alegre (HCPA) from March 2020 to May 2021 with Severe Acute Respiratory Syndrome (SARS) from COVID-19 and underwent CTPA. HCPA is a University, teaching hospital and tertiary care facility. The study was approved by the HCPA Research Ethics Committee (n° 27559019.3.0000.5327). Patients' informed consent was waived due to its retrospective nature.
The electronic medical records of all COVID-19 patients who had clinical suspicion of PTE and underwent CTPA were reviewed. SARS from COVID-19 was defined as a patient with a positive result in RT-PCR (real-time reverse transcriptase-polymerase chain reaction) or antigen testing (immunochromatography); at least two of the signs and symptoms ‒ sudden onset fever, chills, headache, cough, runny nose, sore throat or problems with smell or taste; and who develops dyspnea, a feeling of heaviness or pressure in the chest, oxygen saturation < 95% or cyanosis.
General clinical data were collected on demographic characteristics, medical history, laboratory tests, CTPA and outcomes during hospitalization. Laboratory results and clinical data related to CTPA were only considered if the interval between CTPA exams and processing of laboratory data was less than 48 hours. Serum D-dimer levels were evaluated using an automated particle-enhanced quantitative immunoturbidimetric assay (Innovance D-DIMER, Siemens Medical Solutions Diagnostics, Deerfield, IL, USA).
Statistical analyses were performed using the Statistical Package for the Social Sciences, version 20.0® (Cary, EUA). Patients were divided into subgroups according to the presence of pulmonary embolism or not according to CTPA results. A descriptive analysis of the characteristics of both groups was performed. Normal distribution was checked by a histogram and by the Shapiro-Wilk test. Descriptive data were expressed as frequencies (%) for categorical data, means and Standard Deviations (SD) for continuous data with normal distribution and median and Interquartile Range (IQ) for continuous data without normal distribution. When appropriate, comparisons between groups were performed using Student's t-test or Mann-Whitney's test, for continuous variables, and Chi-Square test or Fisher's exact test for categorical variables. The analysis of factors associated with pulmonary embolism was performed by multivariate robust Poisson Regression. The multivariate model was built through a backward stepwise selection. Confounding variables were selected based on their association with the dependent variable in the univariate analysis (p < 0.1) and their presumed causal association with the outcome. Variables with missing data above 10% were excluded from the multivariate analysis. Statistical significance was accepted at p < 0.05.
The ability of D-dimer collected within 48 hours of the CTPA to classify patients with or without PTE according to CT angiography was evaluated with statics thresholds of 0.3 μg/mL or more, and also with an age-adjusted threshold [0.01 × (age – 50 years)] for patients aged over 50 years. Receiver Operator Characteristics (ROC) curve was built.
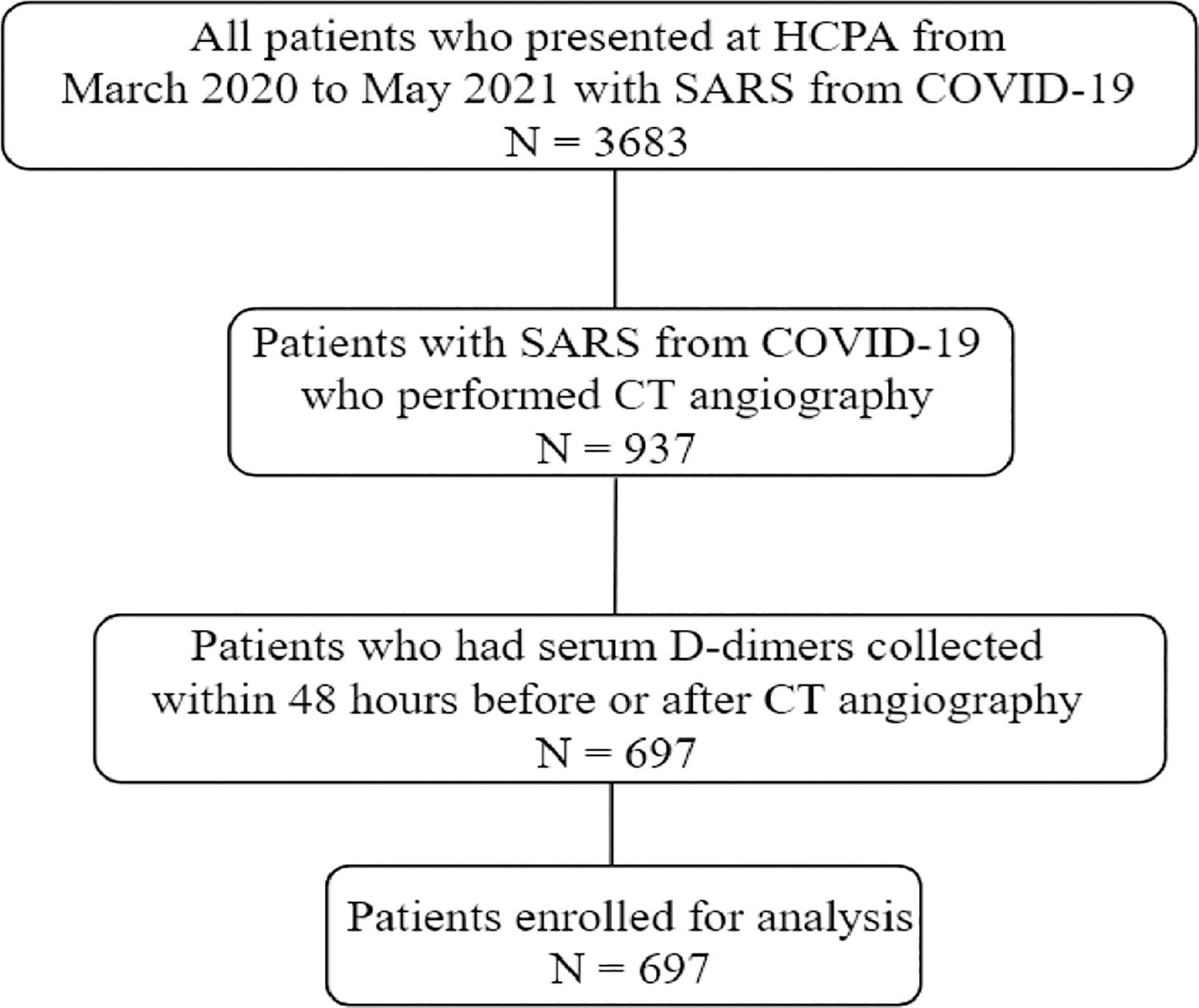
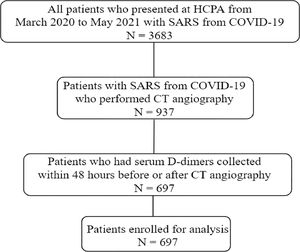
ResultsA total of 3,683 patients who met diagnostic criteria for SARS from COVID-19 were hospitalized from March 2020 to May 2021. Pulmonary CT angiograms were performed in 937/3683 (25%) patients. Among patients who underwent CT angiography, 697 had available serum D-dimers collected within 48 hours of the exam and were enrolled for final analysis (Fig. 1). The excluded patients did not differ from the included patients in terms of age, sex, comorbidities, intensive care unit length of stay, need for mechanical ventilation, or death (data not shown, p < 0.05).
Among 697 patients with COVID-19 and suspected PTE [382 (54.8%) men; mean (SD) age, 59 (20.5) years], 499 (71.5%) patients required intensive care and 248 (35.6%) patients died during hospitalization. Of 697 patients who underwent CTPA, 226 (32.4%) patients had radiographic evidence of PTE (chest computed tomography), of which 122 (54%) were segmental, 44 (19.6%) lobar, 21 (14.1%) subsegmental and 28 (12.3%) proximal.
PTE-positive and PTE-negative group comparisonThe demographic, laboratory and clinical features at baseline, pre-existing conditions, and outcome data for each group are shown in Table 1. Patients’ clinical and laboratory characteristics by CTPA and CTPA findings are shown in Table 2.
Baseline clinical and laboratory features of the patients with COVID-19 and hospitalization data.
| Total (n = 697) | Pulmonary embolism present (n = 226) | Pulmonary embolism absent (n = 471) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| N° of patients (%) | ||||
| Sexc | 0.256 | |||
| Male | 382 (54.8) | 131 (57.9) | 251 (53.3) | |
| Female | 315 (45.1) | 95 (42.1) | 220 (46.7) | |
| Age (years)b | 59 (47; 67.5) | 61 (47;68) | 58 (47;67) | 0.562 |
| Body Mass Index (kg/m²)b | 29,4 (8.3) | 29,3 (9.1) | 29,4 (8.1) | 0.722 |
| Comorbidities | ||||
| N° of patients (%) | ||||
| Hypertensionc | 389 (55.8) | 124 (54.8) | 265 (56.2) | 0.745 |
| Diabetes mellitusc | 212 (30.4) | 71 (31.4) | 141 (29.9) | 0.725 |
| Chronic kidney diseasec | 76 (10.9) | 18 (7.9) | 58 (12.3) | 0.092 |
| Renal replacement therapy (previous)c | 41 (5.8) | 11 (4.8) | 30 (6.3) | 0.494 |
| Cerebrovascular diseasec | 39 (5.6) | 14 (6.2) | 25 (5.3) | 0.725 |
| Liver diseasec | 9 (1.3) | 3 (1.3) | 6 (1.3) | 1.000 |
| Heart diseasec | 87 (12.5) | 26 (11.5) | 61 (12.9) | 0.626 |
| Neurological diseasec | 24 (3.4) | 12 (5.3) | 12 (2.5) | 0.075 |
| COPDc | 46 (6.6) | 13 (5.7) | 33 (7) | 0.626 |
| Asthmac | 42 (6) | 10 (4.4) | 32 (6.8) | 0.239 |
| Smoking (present or past)c | 163 (23.4) | 51 (22.5) | 112(23.8) | 0.775 |
| Malignancyc | 51 (7.3) | 11 (5) | 40 (8.5) | 0.090 |
| Use of immunosuppressantc | 38 (5.5) | 11 (4.9) | 27 (5.9) | 0.724 |
| Transplantedc | 25 (3.6) | 5 (2.2) | 20 (4.2) | 0.199 |
| HIVc | 15 (2.2) | 2 (0.8) | 13 (2.7) | 0.162 |
| Laboratory baseline findings | ||||
| D-dimer (μg/mL)b | 1.4 (0.7; 3.6) | 1.9 (0.7; 12.8) | 1.3 (0.6; 2.4) | 0.000 |
| White blood cell count (103/μL)b | 8.5 (6.3; 12) | 9 (6.6; 12.6) | 8.3 (6.2; 11.3) | 0.058 |
| Lymphocytes (103/μL)b | 0.8 (0.5; 1.1) | 0.7 (0.5; 1) | 0.8 (0.6; 1.1) | 0.021 |
| Hemoglobin (g/dL)b | 12.9 (11.7; 14.2) | 12.9 (11.9; 14.1) | 12.9 (11.6; 14.2) | 0.817 |
| Platelet count (103/μL)b | 207 (158; 276.3) | 219.5 (166; 269) | 202.5 (154; 282) | 0.226 |
| Lactate dehydrogenase (U/L)b | 473.5 (356; 637) | 530 (413; 732) | 436.5 (333; 603) | 0.000 |
| Lactate (mmoL/L)b | 1.4 (1; 1.8) | 1.5 (1.2; 1.9) | 1.3 (1; 1.7) | 0.003 |
| Prothrombin time (INR)b | 1.1 (1; 1.2) | 1.1 (1; 1.2) | 1 (1; 1.1) | 0.000 |
| Partial thromboplastin time (seconds)b | 35 (31.2; 39.6) | 34.7 (31.3; 39.4) | 35 (31.1; 39.7) | 0.605 |
| Creatinine (mg/dL)b | 1 (0.8; 1.4) | 1 (0.8; 1.4) | 1 (0.8; 1.4) | 0.826 |
| Troponin I US (ng/mL)b | 10 (10; 28.6) | 13.6 (10; 48) | 10 (10; 19.9) | 0.000 |
| Creatine kinase (U/L)b | 108 (57; 269.5) | 147.5 (67.8; 370) | 97 (53; 231) | 0.002 |
| Total bilirubin (mg/dL)b | 0.5 (0.4; 0.7) | 0.5 (0.4; 0.8) | 0.5 (0.4; 0.7) | 0.026 |
| Fibrinogen (mg/L)b | 631 (542; 733) | 635 (545; 737) | 627.5 (541; 733) | 0.990 |
| CRP (mg/L)b | 136.5 (78; 205) | 144.2 (88; 215) | 128.3 (73; 194) | 0.021 |
| Hospitalization data | ||||
| N° of patients (%) | ||||
| ICU hospitalization | 499 (71.5) | 188 (83.1) | 311 (66) | 0.000 |
| Ventilatory support | 0.000 | |||
| Oxygen supplementation | 86 (12.3) | 16 (7.1) | 70 (14.7) | |
| Non-invasive mechanical ventilation | 148 (21.2) | 32 (14.2) | 116 (24.6) | |
| Invasive mechanical ventilation | 434 (62.3) | 174 (77) | 260 (55.2) | |
| Renal replacement therapy (new)c | 226 (32.4) | 47 (20.8) | 88 (18.7) | 0.539 |
| Days of symptoms before admissionb | 8 (5; 11) | 8 (6; 12) | 7 (5; 10) | 0.005 |
| Length of mechanical ventilationb | 16 (9; 26) | 15.5 (10; 25.3) | 16 (8; 26) | 0.944 |
| Length of ICU stayb | 15 (8; 26) | 17 (11; 28) | 13.5 (7; 26) | 0.095 |
| Length of hospital stayb | 21 (11; 32) | 23 (15; 33) | 19 (10; 32) | 0.001 |
| Outcome | ||||
| N° of patients (%) | ||||
| Survivorc | 449 (64.4) | 130 (57.5) | 319 (67.7) | 0.009 |
| Non survivor | 248 (35.6) | 96 (42.5) | 152 (32.3) |
aData expressed as mean (standard deviation) and t-test was performed.
Clinical and laboratory features of the patients with COVID-19 by CTPA and CTPA findings.
| Total (n = 697) | Pulmonary embolism present (n = 226) | Pulmonary embolism absent (n = 471) | p-value | |
|---|---|---|---|---|
| Laboratory findings by CTPA | ||||
| D-dimer (μg/mL)b | 3.46 (1.5; 9.9) | 9.1 (3.9; 20) | 2.3 (1.2; 5.1) | 0.000 |
| White blood cell count (103/μL)b | 10.2 (13.8; 10.2) | 11.1 (8.3; 14.3) | 9.7 (7.2; 13.5) | 0.002 |
| Lymphocytes (103/μL)b | 0.84 (0.5; 1.3) | 0.8 (0.5; 1.1) | 0.9 (0.5;1.3) | 0.012 |
| Hemoglobin (g/dL)b | 11.8 (10.2; 13.4) | 11.5 (10.2; 13) | 12.1 (10.3; 13.4) | 0.050 |
| Platelet count (103/μL)b | 245.5 (182; 321) | 244 (177; 307) | 247 (184; 328) | 0.261 |
| Lactate dehydrogenase (U/L)b | 478 (345; 624) | 532 (419; 764) | 441 (319;600) | 0.000 |
| Prothrombin time (INR)b | 1.1 (1; 1.2) | 1.2 (1.1; 1.3) | 1.1 (1; 1.2) | 0.000 |
| Partial thromboplastin time (s)b | 35.7 (32.2; 41.9) | 37.2 (33.6; 46.2) | 35 (31.2; 40.5) | 0.001 |
| Creatinine (mg/dL)b | 0.9 (0.7; 1.5) | 0.9 (0.7; 1.6) | 0.9 (0.7; 1.4) | 0.929 |
| Troponin I US (ng/mL)b | 10 (10; 37.9) | 18.4 (10; 79.9) | 10 (10; 22.3) | 0.000 |
| Lactate (mmoL/L)b | 1.4 (1.1; 1.8) | 1.5 (1.9; 1.2) | 1.4 (1.1; 1.8) | 0.141 |
| Creatine kinase (U/L)b | 109 (48; 339) | 181 (63; 503) | 87.5 (41; 293) | 0.002 |
| PaO2/FiO2b | 162 (110; 220) | 163.3 (118; 224) | 159.9 (108; 220) | 0.516 |
| Total bilirubin (mg/dL)b | 0.5 (0.4; 0.7) | 0.6 (0.4;0.9) | 0.5 (0.3; 0.7) | 0.097 |
| Fibrinogen (mg/L)a | 624.8 (179.9) | 579 (188.1) | 645.3 (162.7) | 0.005 |
| CRP (mg/L)b | 122.1 (68; 203) | 139 (83;209) | 117.4 (58; 193) | 0.007 |
| Clinical data by CTPA | ||||
| N° of patients (%) | ||||
| Days of hospitalization at CTPAb | 4 (1;8) | 5 (1; 9) | 3 (1; 8) | 0.109 |
| Anticoagulant use before CTPAc | 0.000 | |||
| Prophylactic | 383 (54.9) | 110 (48.7) | 273 (57.9) | |
| Therapeutic | 91 (13.1) | 53 (23.4) | 38 (8.1) | |
| Ventilatory support at CTPAc | 0.000 | |||
| Oxygen supplementation | 207 (29.7) | 41 (18.1) | 166 (35.2) | |
| Non-invasive ventilation | 88 (12.6) | 29 (12.8) | 59 (12.5) | |
| Invasive mechanical ventilation | 343 (49.2) | 145 (64.2) | 198 (42) | |
| Vasopressor at CTPAc | 377 (54.1) | 156 (69) | 221 (46.9) | 0.000 |
| Pulmonary embolism | ||||
| N° of patients (%) | ||||
| Laterality | ||||
| Bilateral | 117 (48.2) | |||
| Unilateral most proximal affected artery | 109 (51.8) | |||
| Proximal (trunk or main) | 28 (12.4) | |||
| Obar | 44 (19.5) | |||
| Segmental | 122 (54) | |||
| Subsegmental | 32 (14.1) |
Age, sex, body mass index and comorbidities showed no significant statistical differences. By univariate analysis, several laboratorial variables (white blood cell count, lymphocytes count, hemoglobin, lactate dehydrogenase, lactate, prothrombin time, high-sensitivity troponin-I, creatine kinase, total bilirubin at admission, C-reactive protein, fibrinogen), most of them known as markers of disease severity, as well as ventilatory support at CTPA and vasopressor use at CTPA were different between PTE-positive and PTE-negative groups, but these differences did not remain after multivariate adjustment.
By multivariate analysis (Table 3), statistically significant differences were found between the PTE-positive and PTE-negative groups for D-dimer levels at admission and at the time of CTPA. The median (p25; p75) D-dimer values at CTPA were 9.1 (3.9; 20) μg/mL for patients with PTE and 2.3 (1.2; 5.1) μg/mL for patients without PTE (adjusted p < 0.001). The median (p25; p75) D-dimer values at admission were 1.88 (0.7; 12.8) μg/mL for patients with PTE and 1.29 (0.6; 2.4) μg/mL for patients without PTE (adjusted p = 0.001). In addition, the use of anticoagulants at a therapeutic dose before CTPA was more frequent among patients with PTE on CTPA [53 (23.4%) vs. 38 (8.1%); adjusted p = 0.001]. PTE was independently associated with higher mortality [96 (42.5%) vs. 152 (32.3%); adjusted p = 0.038] and need for mechanical ventilation [174 (77%) vs. 260 (55.2%); p < 0.001] and ICU admission [188 (83.1%) vs. 311 (66%); p < 0.001] during hospitalization.
Multivariate analysis for factors associated with pulmonary embolism in COVID-19.
Data expressed as relative risk (confidence interval). Adjusted by robust Poisson regression model.
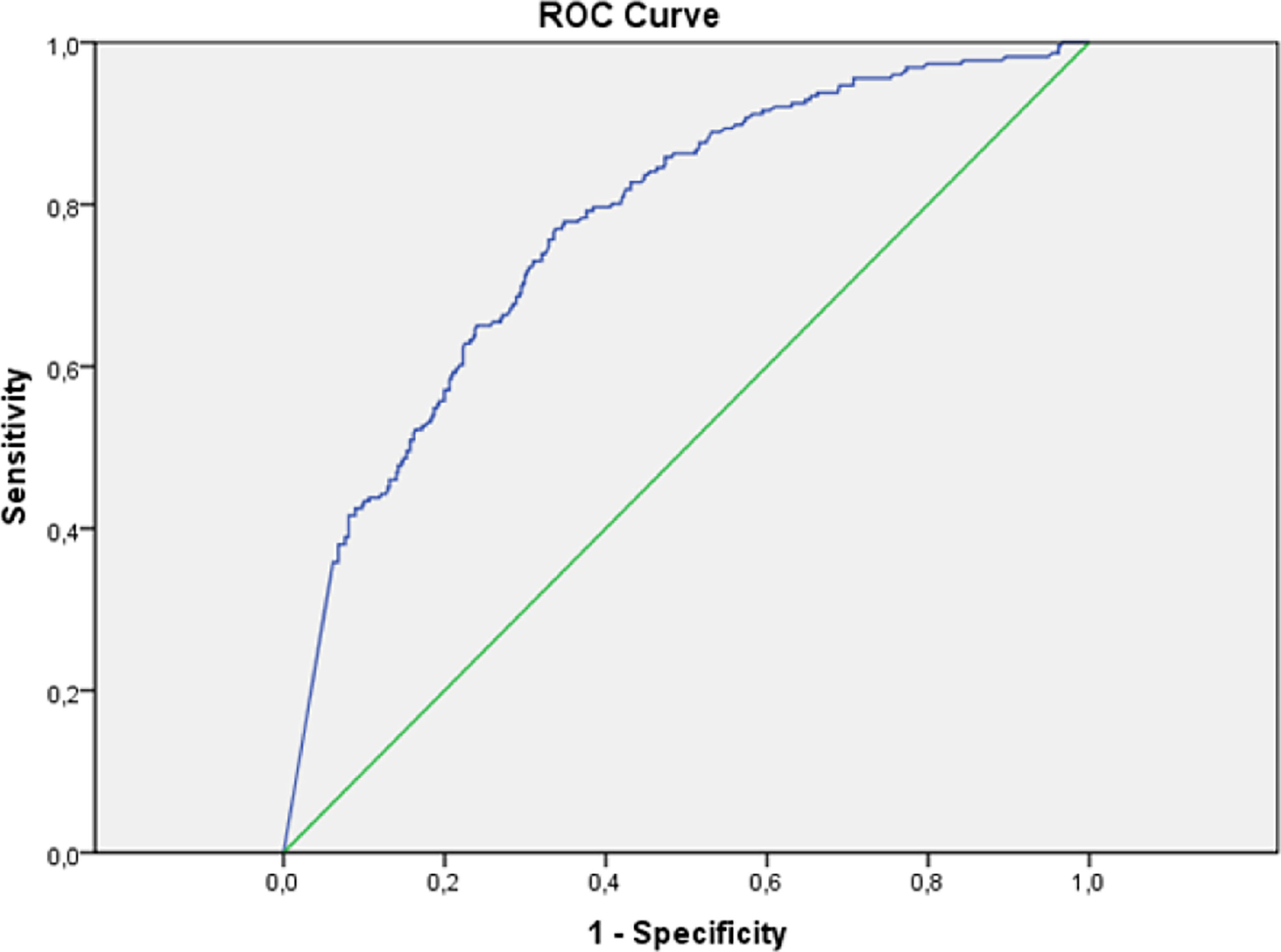
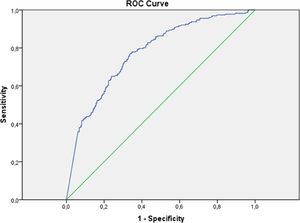
Performance measures for D-dimer thresholds are presented in Table 4. A D-dimer concentration of 0.5 μg/mL or above was associated with a sensitivity of 98.2%, specificity of 5.7%, Negative Predictive Value (NPV) of 87.1%, Positive Predictive Value (PPV) of 33.3%, with 95.6% of patients with increased values. The age-adjusted interpretation strategy for D-dimers (< 50 years 0.5 μg/mL; ≥ 50 years 0.01 × age μg/mL) resulted in a sensitivity of 98.2% and specificity of 8.9%. Using the static threshold of 0.5 μg/mL, four pulmonary embolisms were missed: three segmental and one lobar. The static threshold of 0.3 μg/mL was associated with 100% of sensitivity, with which 99.1% of patients had increased values. ROC analyses demonstrated D-dimer levels had moderate discriminative power to detect PTE, with an area under the curve (AUC) of 0.77 (Fig. 2).
D-dimer performance measures to detect pulmonary embolism diagnosed by CTPA in COVID-19 patients.
PPV, Positive Predictive Value; NPV, Negative Predictive Value.
Regarding the statistical power to assess D-dimer sensitivity and specificity to detect PTE, for a period-prevalence of 32.4% of PTE, our sample of 697 individuals submitted to D-dimer testing and CTPA provides a statistical power of 81.9% and an alpha error of 4%.32
DiscussionThis retrospective study included 697 patients with COVID-19 that underwent CTPA due to PTE clinical suspicion. Consistent with some previous studies, the incidence of PTE was 32.4%.2,3,13,18,33 Nevertheless, the incidence of PTE in COVID-19 patients varies widely (6.4%‒57%) in the literature and remains uncertain. Studies in which all patients underwent CTPA, the incidence was 18%‒57%, with a pooled incidence determined by meta-analysis of 30.2% [95% CI: 21.0‒41.3].1
Studies of patients with COVID-19 admitted to the ICU reported higher incidence rates of PTE than did those patients who were not admitted to the ICU.1 Since 71.5% of our sample required intensive care during hospitalization, the overall high level of disease severity in our study population may explain why the incidence found was above that observed in some previous studies. Also, in our study, the presence of PTE was associated with worse prognostic assessment laboratory tests, need for ICU admission and mechanical ventilation during hospitalization, and higher mortality rate (42.5 vs. 32.3, adjusted p = 0.038). This corroborates the correlation between PTE incidence and COVID-19 level of severity.
In line with previous studies, we found that high D-dimer levels are common in COVID-19 patients, even in the absence of PTE. In our sample, D-dimer value was above normal in over 95% of patients [median (IQ) 1.41 μg/mL (0.7; 3.6) at admission; 3.46 μg/mL (1.5; 9.9) at CTPA], being higher among PTE-positive patients [median (IQ) 1.88 (0.7; 12.8) μg/mL vs. 1.29 (0.6; 2.4) μg/mL at admission; 9.1 (3.9; 20) μg/mL vs. 2.3 (1.2; 5.1) μg/mL at CTPA ‒ p < 0.001], in parallel with other studies.34
Because of the high D-dimer levels found in COVID-19 patients even in the absence of PTE, some previous studies have suggested optimal higher D-dimer cutoffs to predict occurrence of PTE at CTPA.33,35,36 Setting higher D-dimer cutoffs improved specificity, but at the cost of reduced sensibitivity, which is unacceptable in a condition such as PTE. Other studies reported new higher cutoffs to assure 100% specificity, which could rule out PTE when used alone.18,37 In this context, D-dimers levels began to be used at some centers to help define management clinical decisions for COVID-19 patients. However, it remains unclear if or how these measures should influence clinical decisions.
In the light of pre-pandemic scientific evidence, we need to remember how D-dimer was used in the clinical decision rules for pulmonary embolism. It is well established that pulmonary embolism can be ruled out if patients with a low to intermediate risk for PTE have a D-dimer level of less than 0.5 μg/mL.26 It includes the inpatient scenario, where, although it kept safe, D-dimer lost efficiency, once the proportion of patients with D-Dimer below the established cut-off was only 8.4%.38 Studies that sought to validate the use of D-dimer to rule out PTE more often used the Wells' Criteria for Pulmonary Embolism to stratify patients' risk. However, Wells' Criteria performance in COVID-19 patients has already been evaluated, and even though four or more points predicted PTE, this outcome was also frequently present with lower scores, behaving in a non-discriminative way when used alone (AUC = 0.54).39
In our study, ROC analyses demonstrated D-dimer had moderate discriminative power to detect PTE, with an AUC of 0.77, similar to the performance found in a meta-analysis that reported an AUC of 0.737 in the summary ROC curve.1 It is important to emphasize that 99.1% of our patients had increased D-dimer values. The threshold of 0.3 μg/mL was associated with 100% sensitivity, which is of no practical use. The usual threshold of 0.5 μg/mL was associated with a sensitivity of 98.2% and a negative predictive value of 87.1%, in contrast to previous studies that found cut-off values for a 100% sensitivity of 2.66 μg/mL18 and 1.6 μg/mL,37 even though they have reported PTE incidences of 25% and 30%, respectively, lower but still relatively high and close to the incidence we found. Another study that included 781 patients who presented to the emergency department, being 56% admitted to the wards and 12% to the ICU, with a PTE incidence of 7.7%, reported that the usual D-dimer threshold of 0.5 μg/mL, as well as the age-adjusted one, could safely rule out PTE in 17.1% and 31.5% of COVID-19 patients, respectively.24 On the other hand, a recently published study in which PTE incidence was 12.9% found that D-dimer levels of 0.5 μg/mL or greater were able to identify all PTE cases in its sample but ruling out PTE in only 7.7% of patients.23
The performance of the strategy that uses D-dimers to exclude PTE depends on pretest probability, which may explain why in our study the use of D dimers was not effective. For patients in whom the risk of PTE is high, a normal D-dimer does not reduce the likelihood of PTE enough to rule out the diagnosis.40,41 Wells' Criteria stratifies PTE risk in two or three categories. The original study reported an incidence of PTE of 1.3%, 16.2% and 37.5% in the low, moderate and high-risk strata, respectively.42 A subsequent validation study found PTE incidences of 2% in low risk, 15% in moderate risk, and 43% in high-risk groups; 3% and 28% for dichotomized classification in “PTE unlikely” and “PTE likely”, respectively.43 Given that in our study the incidence of PTE was 32.4%, our sample would correspond to a classification of high risk in Wells’ Criteria, a clinical situation in which it is already known that D-dimers cannot be used to reliably exclude PTE. Knowing that the incidence of PTE varies according to the severity of COVID-19, it can be speculated that using D-dimers to rule out PTE may eventually be a valid strategy for patients with less severe disease and, therefore, with a lower pretest probability for PTE.
This study had limitations, mainly related to retrospective data collection. First, we only included patients with both D-dimer and CTPA results available, which may have introduced selection bias by excluding patients unable to undergo CTPA or that, given the overlap of symptoms with COVID-19, did not have PTE suspected. Moreover, in the context of COVID-19, D-dimers are routinely ordered to assess prognosis, but we could not be sure if the D-dimer was also being used to predict PTE, which would select patients with higher D-dimers to undergo CTPA. Additionally, retrospective design prevented risk stratification for PTE through the application of the Wells score or another tool and made it difficult to control for confounders that could influence the outcomes. Finally, it should be noted that 68% of patients were receiving heparin at prophylactic or therapeutic doses at the time of PTE diagnosis and that we did not evaluate for other concomitant types of thromboembolism, which may have influenced D-dimer results.
ConclusionsIn conclusion, the current study suggests that, although D-dimer levels are higher among COVID-19 hospitalized patients with PTE as compared to those without PTE, its use to exclude PTE may be unsuitable and have limited clinical utility. If there is a role for D-dimers in the clinical decision rules for pulmonary embolism in patients with COVID-19, possibly it would be along with clinical prediction scores of pretest probability applicable in this specific population. Future studies with prospective testing of D-dimer thresholds and risk stratification methods in patients with COVID-19 are needed to clarify the D-dimer performance and usefulness to rule out PTE in this population.
Ethical approval statementAll procedures performed in this study were in accordance with the ethical standards of the HCPA Research Ethics Committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent statementNot applicable.
Financial supportThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Study conducted at the Hospital de Clínicas de Porto Alegre, Porto Alegre, RS, Brazil.