Multidrug-resistant gram-negative rods (MDR GNR) represent a growing threat for patients with cancer. Our objective was to determine the characteristics of and risk factors for MDR GNR bacteremia in patients with cancer and to develop a clinical score to predict MDR GNR bacteremia.
Material and MethodsMulticenter prospective study analyzing initial episodes of MDR GNR bacteremia. Risk factors were evaluated using a multiple logistic regression (forward-stepwise selection) analysis including variables with a p<0.10 in univariate analysis.
Results394 episodes of GNR bacteremia were included, with 168 (42.6 %) being MDR GNR. Five variables were identified as independent risk factors: recent antibiotic use (OR=2.8, 95 % CI 1.7–4.6, p=0.001), recent intensive care unit admission (OR=2.9, 95 % CI 1.1–7.8, p=0.027), hospitalization ≥ 7 days prior to the episode of bacteremia (OR=3.5, 95 % CI 2–6.2, p=0.005), severe mucositis (OR=5.3, 95 % CI 1.8–15.6, p=0.002), and recent or previous colonization/infection with MDR GNR (OR=2.3, 95 % CI 1.2–4.3, p=0.028). Using a cut-off value of two points, the score had a sensitivity of 66.07 % (95 % CI 58.4–73.2 %), a specificity of 77.8 % (95 % CI 71.4–82.7 %), a positive predictive value of 68 % (95 % CI 61.9–73.4 %), and a negative predictive value of 75.9 % (95 % CI 71.6–79.7 %). The overall performance of the score was satisfactory (AUROC 0.78; 95 % CI 0.73-0.82). In the cases with one or none of the risk factors identified, the negative likelihood ratio was 0.18 and the post-test probability of having MDR GNR was 11.68 %.
ConclusionsWith the growing incidence of MDR GNR as etiologic agents of bacteremia in cancer patients, the development of this score could be a potential tool for clinicians.
Bloodstream infections still represent one of the major complications in patients with cancer1,2 and patients receiving a hematopoietic stem cell transplant (HSCT).3,4 They are associated with prolonged hospitalization, higher healthcare costs, and significant mortality.1,4
The etiological spectrum of bacteria causing bacteremia in this population has fluctuated with time. Initially, Gram-negative rods (GNR) were the most frequent, then it shifted to Gram-positive cocci (GPC), and in the last years, there has been an alarming increase in Multidrug-Resistant (MDR) organisms,1,4–7 which are associated with higher morbidity and mortality.1,4–7 MDR GNR are a matter of concern worldwide.1,4,8,9 This group is usually comprised by Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBL-E), carbapenemases (CPE) (especially KPC) and Amp-C cephalosporinases; and also MDR non-fermenters such as MDR Pseudomonas aeruginosa (MDR-PSA), MDR Stenotrophomonas maltophilia (MDR-Steno) and MDR Acinetobacter baumannii (MDR-Acineto).5,8,9
Treatment options for MDR GNR are limited,7,10 and in patients with cancer and neutropenia, where the recommended empirical treatment for fever in national11 and international12 guidelines is usually piperacillin/tazobactam or cefepime, the presence of MDR-GNR bacteremia conveys a higher frequency of inadequate treatments with higher mortality.4,13,14
New molecular and microbiological methods for early detection of MDR GNR are being studied, evaluated and used in parts of the world,9,15 but not in Argentina. Therefore, clinicians must choose the best antibiotic therapy on empirical basis. Thus, the risk of inadequate empirical therapy should be counterbalanced by unnecessary use of broad-spectrum antibiotics, such as carbapenems. Identification of risk factors for MDR GNR bacteremia and the subsequent design of a score to detect those patients that would potentially benefit from broader spectrum antibiotics could be a useful tool for clinicians.
Multiple studies have outlined the risk factors for MDR GNR.4,16–18 However, no clinical score has been designed to include both cancer patients with GNR bacteremia and local data. Furthermore, different incidence, resistance patterns and risk factors associated with MDR GNR have been reported worldwide due to the current changes in epidemiology.19 As information on this topic is scarce in Argentina,20,21 we decided to carry out this multicenter study to determine the characteristics and risk factors for MDR GNR bacteremia in patients with cancer or HSCT and to further design a clinical score to predict MDR GNR bacteremia.
Materials and methodsSetting, patients and study designMulticenter prospective study performed in 10 referral centers specialized in the assistance of oncological and transplant patients in Argentina. Patient characteristics and the medical treatment provided were similar in these centers. Our objective was to determine the characteristics of and risk factors for MDR GNR bacteremia in patients with cancer and to develop a clinical score to predict MDR GNR bacteremia.
All episodes of initial bacteremia (defined as the first episode of bacteremia experienced during an admission) caused by GNR in adults patients (≥ 18 years of age) managed as inpatients from May 2014 to November 2016 were included, in patients with: a) solid or hematological malignancies treated with chemotherapy or biological agents (six months prior to admission), or if they had been receiving steroids (dose equal or higher to prednisone 20mg daily or equivalent, for at least two weeks prior to admission); or b) HSCT patients, either allogeneic (with graft versus host disease at any time or without this disease in the first two years) or autologous (in the first year post-transplant). We excluded from the analysis patients already enrolled in the study, those with bacteremias who refused to participate in the study, and patients receiving palliative care. Patients were identified at the time of positive blood culture and then prospectively followed. Data were obtained from medical records (electronic and paper records depending on the center involved) and direct patient care, with a double check made with microbiological records from the laboratory. Clinical, microbiological, treatment, and outcome variables were evaluated. An empirical personalized antibiotic therapy was started based on the patient’s clinical and epidemiological features, and at the discretion of the attending physician. Patients were followed for either 30 days after the episode (by direct patient care in patients still hospitalized or by a phone call in patients discharged) or until the patient’s death, if it happened before (assessed by direct patient care in patients still hospitalized or by a local healthcare database in each center).
DefinitionsNeutropenia was defined as an absolute neutrophil count < 500 cells/mm3. High-risk febrile neutropenia was defined according to clinical variables and a Multinational Association for Supportive Care in Cancer (MASCC) score of < 21.12 The clinical source of infection was determined based on the isolation of the bacteria in the suspected source and/or the associated clinical signs and symptoms. Recent antibiotic use was defined as any antibiotic used 30 days before the episode of bacteremia and for more than 48h. Recent intensive care unit (ICU) admission was defined as an admission within 14 days prior to the episode of bacteremia and for at least 72h. A central venous catheter was determined as a risk factor when it had been in place for at least 72h before the episode of bacteremia. Colonization or infection with an MDR GNR was defined as previous when it happened within six months before hospitalization, whereas colonization with an MDR GNR was defined as recent when it was detected within one week of the episode of bacteremia.
Bacteremia was classified as nosocomial, health-care-associated, or community-acquired according to Friedman, et al.22 Breakthrough bacteremia was defined as an episode of continuous or new-onset bacteremia in a patient receiving appropriate antibiotics for the microorganism recovered from blood cultures. The empirical antibiotic treatment (EAT) was adequate provided that it consisted of one or more antibiotics active in vitro against the isolated bacteria. In the case of patients with ESBL-E, empirical therapy with piperacillin/tazobactam or cefepime alone was considered inadequate.23 Response to treatment on day 7 of therapy was defined as absence of fever and hypotension, and clinical improvement. Mortality was related to infection provided that there was microbiological, histological, or clinical evidence of active infection.
Microbiological studiesBacteremia was defined as the isolation of a pathogenic bacteria in at least one bottle of blood culture (BD BACTEC F Aerobic and Anaerobic, analyzed with BACTEC FX BD, BacTALERT 3D Biomerieux or BRITANIA depending on method available at each center), for a minimum incubation period of five days. In the case of GNR other than Enterobacteriaceae or Pseudomonas aeruginosa, two positive bottles were required in order to be defined as a true bloodstream infection. MDR GNR were defined as a GNR resistant to three or more of the following categories of antibiotics: carbapenems, piperacillin/tazobactam, 3rd and 4th generation cephalosporins, aztreonam, fluoroquinolones, or aminoglycosides.24,25 All S. maltophilia isolates were considered MDR. Microbiological identification and susceptibility testing were done with manual biochemical and microbiological methods, disk diffusion with halo comparison, and using Etest, VITEK II Complex from Biomereux, PHOENIX 100 BD, and MALDI-TOF (Microflox from Bruker), according to the recommendations of the CLSI.26 Carbapenemase production was investigated in carbapenem-resistant bacteria using the modified Hodge method, the Ertapenem susceptibility test by disk diffusion and the Boronic Acid Disk test for KPC, and the Imipenem-EDTA Double-Disk Synergy Test to identify Metallo-β-lactamases. The presence of genes coding for major carbapenemases (i.e. blaVIM, blaNDM, blaIMP, blaKPC and blaOXA-48) was investigated by polymerase chain reaction using specific primers for some isolates. Rectal swabs to detect colonization with KPC, ESBL and MDR PSA were routinely collected (once a week and in every pre-transplant evaluation) in six of the 10 centers involved in the study using chromogenic methods.
Statistical analysisTo compare the characteristics, treatments, and outcomes of patients with MDR GNR and non-MDR GNR, the χ2 test, Fisher’s exact test and Mann-Whitney U-test were used when indicated. To identify the risk factors for MDR GNR that were included as variables in the score, a multiple logistic regression model (forward-stepwise selection) was used. Variables with a p<0.10 in univariate analysis were included in the multivariate model. For multivariate analysis, variables with a statistically significant association at p<0.05 were used to develop the score. A tetrachoric correlation model was used to evaluate collinearity. The calibration of the model was evaluated using the Hosmer-Lemeshow test, and the Nagelkerke and Cox/Snell R2 were calculated. The predictive performance of the model and the score was evaluated using sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the ROC curve (AUROC), with a value of 1.0 indicating perfect prediction. Post-test probability of the different values of the score to predict for MDR GNR was further assessed, with a pre-test probability of 42 % (data obtained from previous local, non-published analysis). Internal validation of the final model was assessed using the bootstrap re-sampling technique. For all tests, a 95 % level of statistical significance was used. All tests were two-tailed. The analyses were performed with the SPSS and Stata 14.0 (Statacorp; USA) software packages.
ResultsA total of 394 episodes of GNR bacteremia of 394 patients were included; 245 patients (62.2 %) had hematological malignancies (acute leukemia, 55.8 %, and lymphoma, 26.2 %, being the most frequent), 77 (19.5 %) had solid tumors, and 72 (18.3 %) had undergone HSCT (52.8 % allogeneic). Thirty-six (9.1 %) were previously colonized with MDR GNR (KPC, 47.2 %, and ESBL, 33.3 %, being the most common), 31 (7.9 %) had a previous infection with MDR GNR (mainly bacteremia and urinary tract infection), and 35 (8.9 %) were colonized with an MDR GNR during the week of the bacteremia episode (ESBL and KPC were the most common). Of the total cohort, 73 (18.5 %) had a recent or previous colonization/infection with an MDR GNR.
One hundred and seventy-nine (45.4 %) patients had recently received antibiotics, piperacillin/tazobactam (47.5 %) and carbapenems (40.8 %) were the most common, and 277 (70.3 %) had neutropenia, 91.7 % classified as high-risk, with a median duration of 13 days. In 287 (72.8 %) a clinical source of infection was identified, 38.3 % abdominal and 18.1 % central venous catheter; and 66 % were classified as nosocomial infections.
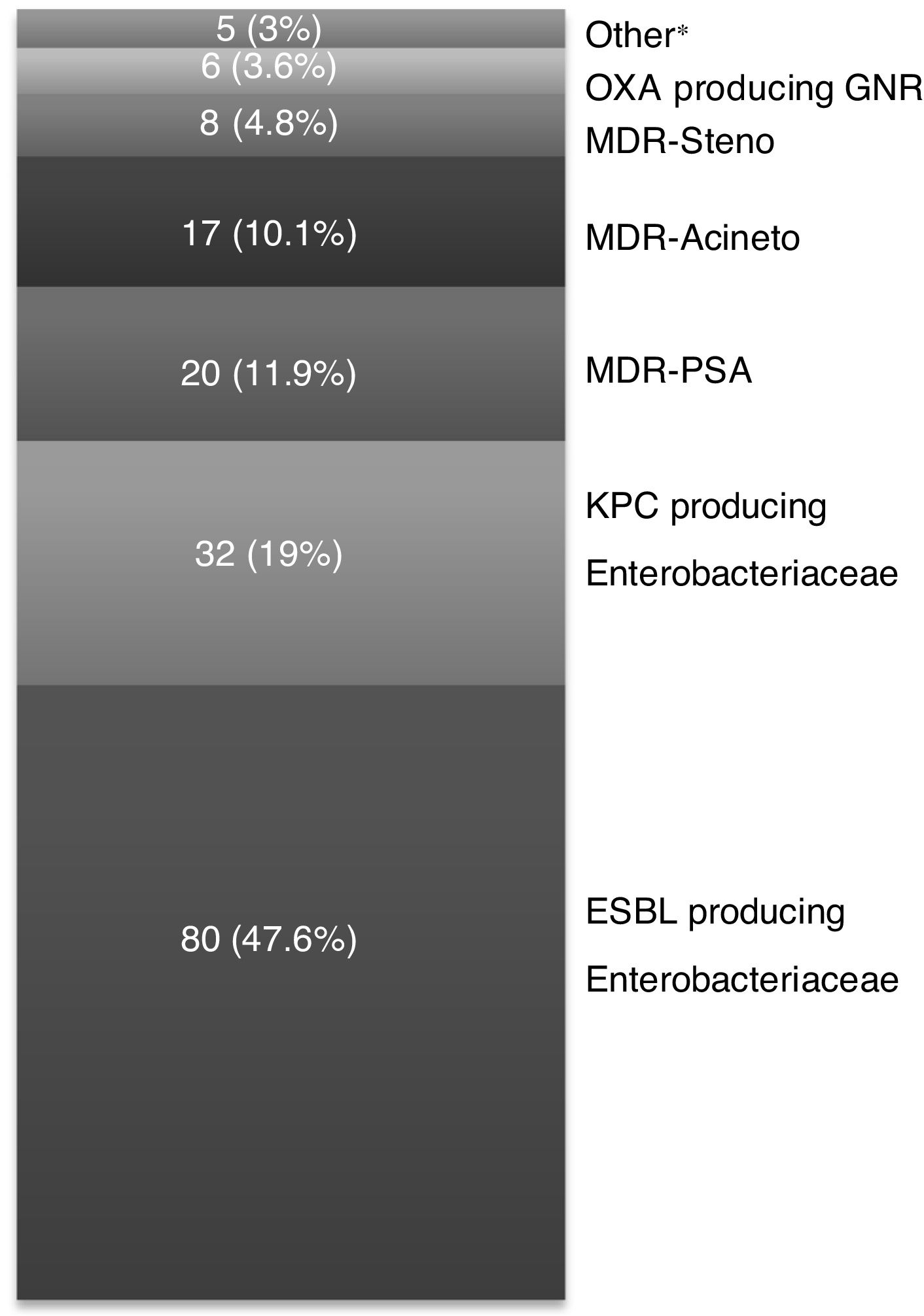
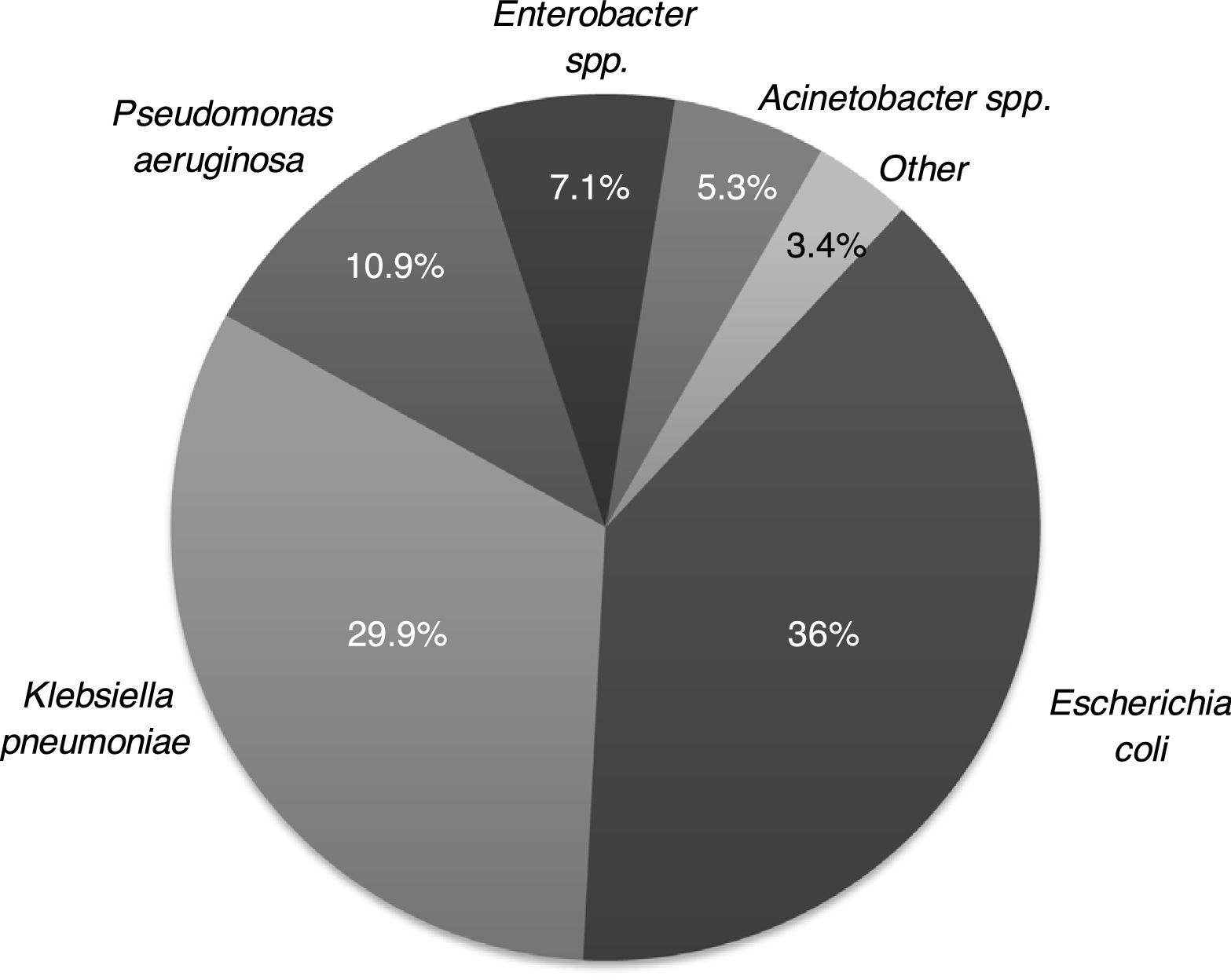
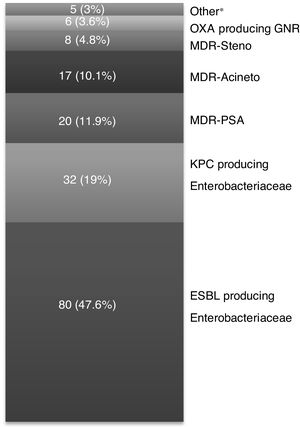
The microbiological characteristics of the isolated GNR are described in Appendix A (Fig. A1). A total of 168 (42.6 %) GNR were classified as MDR GNR, with ESBL-E and CPE (KPC) being the most frequent (Fig. 1). The main differences between the MDR GNR isolated are highlighted in Appendix A (Table A1). Thirty-six episodes (9.1 %) had polymicrobial bacteremia. No outbreaks due to MDR GNR were observed during the study period.
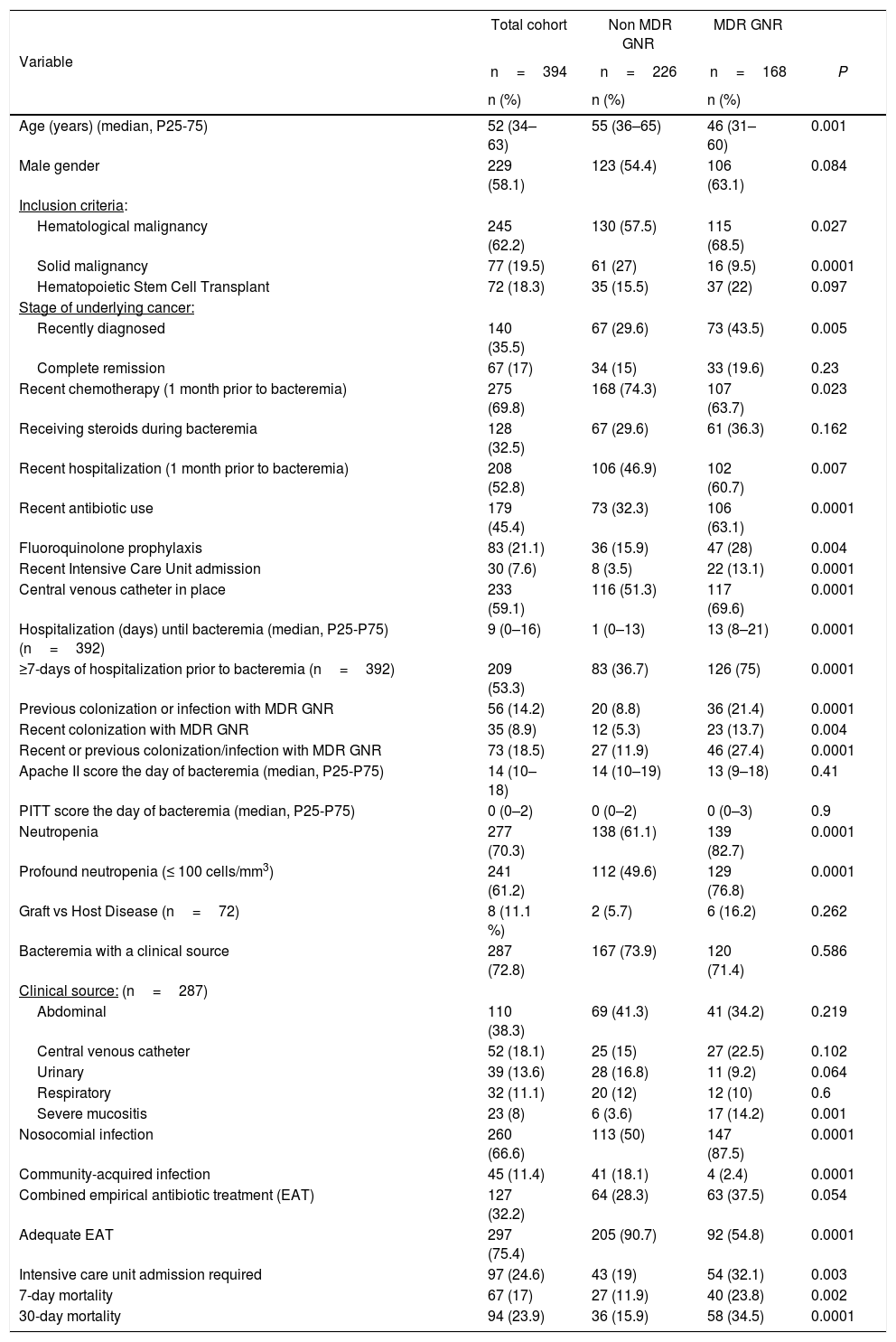
Differences between non-MDR GNR (226, 57.4 %) and MDR GNR (168, 42.6 %) are detailed in Table 1.
Characteristics of the cohort and differences between patients with non-MDR GNR and MDR GNR (n=394).
| Variable | Total cohort | Non MDR GNR | MDR GNR | |
|---|---|---|---|---|
| n=394 | n=226 | n=168 | P | |
| n (%) | n (%) | n (%) | ||
| Age (years) (median, P25-75) | 52 (34–63) | 55 (36–65) | 46 (31–60) | 0.001 |
| Male gender | 229 (58.1) | 123 (54.4) | 106 (63.1) | 0.084 |
| Inclusion criteria: | ||||
| Hematological malignancy | 245 (62.2) | 130 (57.5) | 115 (68.5) | 0.027 |
| Solid malignancy | 77 (19.5) | 61 (27) | 16 (9.5) | 0.0001 |
| Hematopoietic Stem Cell Transplant | 72 (18.3) | 35 (15.5) | 37 (22) | 0.097 |
| Stage of underlying cancer: | ||||
| Recently diagnosed | 140 (35.5) | 67 (29.6) | 73 (43.5) | 0.005 |
| Complete remission | 67 (17) | 34 (15) | 33 (19.6) | 0.23 |
| Recent chemotherapy (1 month prior to bacteremia) | 275 (69.8) | 168 (74.3) | 107 (63.7) | 0.023 |
| Receiving steroids during bacteremia | 128 (32.5) | 67 (29.6) | 61 (36.3) | 0.162 |
| Recent hospitalization (1 month prior to bacteremia) | 208 (52.8) | 106 (46.9) | 102 (60.7) | 0.007 |
| Recent antibiotic use | 179 (45.4) | 73 (32.3) | 106 (63.1) | 0.0001 |
| Fluoroquinolone prophylaxis | 83 (21.1) | 36 (15.9) | 47 (28) | 0.004 |
| Recent Intensive Care Unit admission | 30 (7.6) | 8 (3.5) | 22 (13.1) | 0.0001 |
| Central venous catheter in place | 233 (59.1) | 116 (51.3) | 117 (69.6) | 0.0001 |
| Hospitalization (days) until bacteremia (median, P25-P75) (n=392) | 9 (0–16) | 1 (0–13) | 13 (8–21) | 0.0001 |
| ≥7-days of hospitalization prior to bacteremia (n=392) | 209 (53.3) | 83 (36.7) | 126 (75) | 0.0001 |
| Previous colonization or infection with MDR GNR | 56 (14.2) | 20 (8.8) | 36 (21.4) | 0.0001 |
| Recent colonization with MDR GNR | 35 (8.9) | 12 (5.3) | 23 (13.7) | 0.004 |
| Recent or previous colonization/infection with MDR GNR | 73 (18.5) | 27 (11.9) | 46 (27.4) | 0.0001 |
| Apache II score the day of bacteremia (median, P25-P75) | 14 (10–18) | 14 (10–19) | 13 (9–18) | 0.41 |
| PITT score the day of bacteremia (median, P25-P75) | 0 (0–2) | 0 (0–2) | 0 (0–3) | 0.9 |
| Neutropenia | 277 (70.3) | 138 (61.1) | 139 (82.7) | 0.0001 |
| Profound neutropenia (≤ 100 cells/mm3) | 241 (61.2) | 112 (49.6) | 129 (76.8) | 0.0001 |
| Graft vs Host Disease (n=72) | 8 (11.1 %) | 2 (5.7) | 6 (16.2) | 0.262 |
| Bacteremia with a clinical source | 287 (72.8) | 167 (73.9) | 120 (71.4) | 0.586 |
| Clinical source: (n=287) | ||||
| Abdominal | 110 (38.3) | 69 (41.3) | 41 (34.2) | 0.219 |
| Central venous catheter | 52 (18.1) | 25 (15) | 27 (22.5) | 0.102 |
| Urinary | 39 (13.6) | 28 (16.8) | 11 (9.2) | 0.064 |
| Respiratory | 32 (11.1) | 20 (12) | 12 (10) | 0.6 |
| Severe mucositis | 23 (8) | 6 (3.6) | 17 (14.2) | 0.001 |
| Nosocomial infection | 260 (66.6) | 113 (50) | 147 (87.5) | 0.0001 |
| Community-acquired infection | 45 (11.4) | 41 (18.1) | 4 (2.4) | 0.0001 |
| Combined empirical antibiotic treatment (EAT) | 127 (32.2) | 64 (28.3) | 63 (37.5) | 0.054 |
| Adequate EAT | 297 (75.4) | 205 (90.7) | 92 (54.8) | 0.0001 |
| Intensive care unit admission required | 97 (24.6) | 43 (19) | 54 (32.1) | 0.003 |
| 7-day mortality | 67 (17) | 27 (11.9) | 40 (23.8) | 0.002 |
| 30-day mortality | 94 (23.9) | 36 (15.9) | 58 (34.5) | 0.0001 |
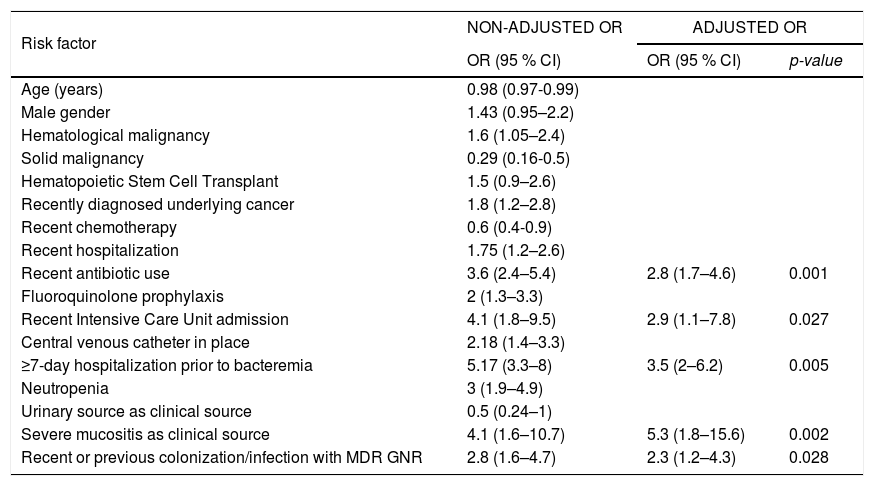
In multivariate analysis (Table 2) five risk factors for MDR GNR remained independently associated: recent antibiotic use (OR=2.8, 95 % CI 1.7–4.6, p=0.001), recent intensive care unit admission (OR=2.9, 95 % CI 1.1–7.8, p=0.027), hospitalization ≥ 7 days prior to the episode of bacteremia (OR=3.5, 95 % CI 2–6.2, p=0.005), severe mucositis (OR=5.3, 95 % CI 1.8–15.6, p=0.002), and recent or previous colonization/infection with MDR GNR (OR=2.3, 95 % CI 1.2–4.3, p=0.028).
Multivariate analysis of the risk factors for MDR GNR bacteremia.
| Risk factor | NON-ADJUSTED OR | ADJUSTED OR | |
|---|---|---|---|
| OR (95 % CI) | OR (95 % CI) | p-value | |
| Age (years) | 0.98 (0.97-0.99) | ||
| Male gender | 1.43 (0.95–2.2) | ||
| Hematological malignancy | 1.6 (1.05–2.4) | ||
| Solid malignancy | 0.29 (0.16-0.5) | ||
| Hematopoietic Stem Cell Transplant | 1.5 (0.9–2.6) | ||
| Recently diagnosed underlying cancer | 1.8 (1.2–2.8) | ||
| Recent chemotherapy | 0.6 (0.4-0.9) | ||
| Recent hospitalization | 1.75 (1.2–2.6) | ||
| Recent antibiotic use | 3.6 (2.4–5.4) | 2.8 (1.7–4.6) | 0.001 |
| Fluoroquinolone prophylaxis | 2 (1.3–3.3) | ||
| Recent Intensive Care Unit admission | 4.1 (1.8–9.5) | 2.9 (1.1–7.8) | 0.027 |
| Central venous catheter in place | 2.18 (1.4–3.3) | ||
| ≥7-day hospitalization prior to bacteremia | 5.17 (3.3–8) | 3.5 (2–6.2) | 0.005 |
| Neutropenia | 3 (1.9–4.9) | ||
| Urinary source as clinical source | 0.5 (0.24–1) | ||
| Severe mucositis as clinical source | 4.1 (1.6–10.7) | 5.3 (1.8–15.6) | 0.002 |
| Recent or previous colonization/infection with MDR GNR | 2.8 (1.6–4.7) | 2.3 (1.2–4.3) | 0.028 |
Hosmer-Lemeshow test value was p=0.72. Nagelkerke and Cox/Snell R2 were 0.36 and 0.27, respectively.
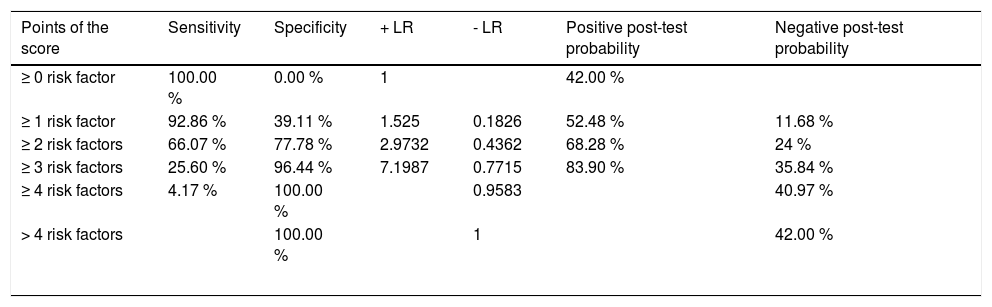
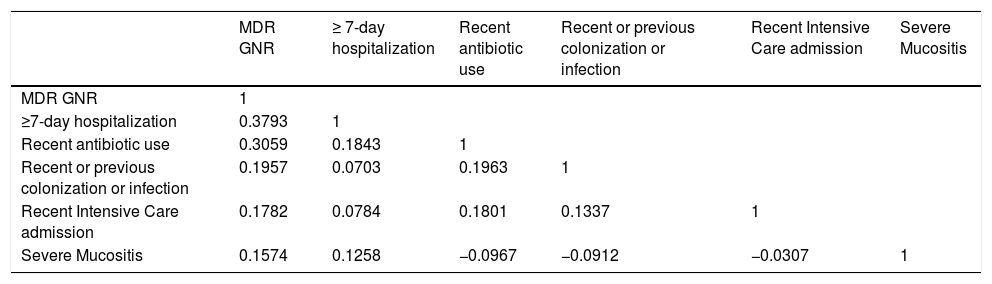
To predict MDR GNR bacteremia, a score was developed using the five variables identified in multivariate analysis, and the presence of each variable was assigned one point. Table 3 shows sensitivity, specificity, likelihood ratios (LR) and post-test probability of MDR GNR bacteremia for each cut-off point of the score. The number of episodes with the different cut-off points of the score is displayed in Table A2 in Appendix A. No collinearity was observed between these variables (Table A3 in Appendix A). Using a cut-off value of two points, the score had a sensitivity of 66.07 % (95 % CI 58.4–73.2 %), a specificity of 77.8 % (95 % CI 71.4–82.7 %), a PPV of 68 % (95 % CI 61.9–73.4 %), and a NPV of 75.9 % (95 % CI 71.6–79.7 %). Internal validation was performed using the bootstrap resampling technique (1000 samples). We calculated the possible deviation of the ß-coefficients (bias), with their respective confidence intervals, and the results were compared both in calibration and discrimination, finding a very high similarity between them. The overall performance of the score was satisfactory (AUROC 0.78; 95 % CI 0.73-0.82). Compared with episodes of bacteremia with one or none of the risk factors, those with two or more had an OR for MDR GNR bacteremia of 6.68 (95 % CI 4.3–10.4). In the cases when one or none of the risk factors was identified, the negative likelihood ratio was 0.18 and the post-test probability of having MDR GNR was 11.68 %.
Sensitivity, specificity and post-test probability of the different cut-off points of the score.
| Points of the score | Sensitivity | Specificity | + LR | - LR | Positive post-test probability | Negative post-test probability |
|---|---|---|---|---|---|---|
| ≥ 0 risk factor | 100.00 % | 0.00 % | 1 | 42.00 % | ||
| ≥ 1 risk factor | 92.86 % | 39.11 % | 1.525 | 0.1826 | 52.48 % | 11.68 % |
| ≥ 2 risk factors | 66.07 % | 77.78 % | 2.9732 | 0.4362 | 68.28 % | 24 % |
| ≥ 3 risk factors | 25.60 % | 96.44 % | 7.1987 | 0.7715 | 83.90 % | 35.84 % |
| ≥ 4 risk factors | 4.17 % | 100.00 % | 0.9583 | 40.97 % | ||
| > 4 risk factors | 100.00 % | 1 | 42.00 % | |||
Monotherapy was the empirical therapy most frequently used (267, 67.8 %), mainly consisting of piperacillin/tazobactam (49.4 %) and carbapenems (37.1 %). On the other hand, combined treatment was used in 127 (32.2 %) cases, with carbapenems plus colistin being the most common combination. The EAT was adequate in 75.4 % of the cases. Seven- and 30-day mortality was 17 % and 23.9 %, respectively, related to infection in more than 80 % of the cases. Marked differences were observed in treatment and outcomes between the two groups. Carbapenems (35.4 versus 61.9 %, p=0.0001) and colistin (11.9 versus 31.5 %, p=0.0001) were more frequently used in the MDR GNR group, with worse outcomes in this group regarding breakthrough bacteremia (4 versus 14.3 %, p=0.0001), clinical response (75.2 versus 54.2 %, p=0.0001), and 30-day mortality (15.9 versus 34.5 %, p=0.0001).
DiscussionThis prospective multicenter study of bacteremia in both cancer and HSCT patients was carried out in Argentina to help understand the characteristics of GNR bacteremia in this population. It further shows the high frequency of MDR GNR as etiological agents and its association with worse outcomes. The possibility that a bacteremia will be caused by an MDR GNR depends on the clinical and microbiological characteristics herein described. With these variables, a clinical score is proposed which may help clinicians treating these patients to decide which antibiotics should be used in the empirical treatment of bacteremia.
The sample analyzed in this study had some unique features, since it only included initial bacteremia mainly observed in patients with hematological malignancies (62.2 %) and mostly classified as nosocomial (66 %). The significant increase in the proportion of GNR as etiological agents of bacteremia in this population has been largely reported.1,7 In our study GNR bacteremia accounted for 66 % of the total cohort of bacteremia of cancer patients in our national registry (data not published), which were included in the final analysis. A striking rise has been observed in MDR GNR as etiological agents of bacteremia in cancer patients.4,7,9,25 The rate of antimicrobial resistance reported in this study is similar to that described in countries with endemic MDR GNR (specially CPE).4,5,27
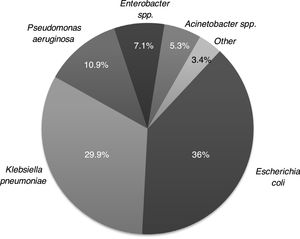
In the analysis of the different groups of MDR GNR, some studies found a significant increase in the frequency of ESBL-E, particularly E. coli and Klebsiella spp, ranging from 12.6 %28 to even 48.3 %.29 In our cohort, ESBL-E proved to be the most frequent MDR GNR, with a 47.6 % frequency. CPE bacteremia has become a matter of concern in cancer patients, with Klebsiella pneumoniae and E. coli7,30 being the most frequent. In our cohort, these microorganisms were the second most common group of MDR GNR (19 %), with a frequency lower than that reported in the literature.30,31 In our study, non-fermenting GNR, such as MDR-PSA, MDR-Acineto and MDR-Steno were the third most frequent group (with 11.9 %, 10.1 % and 4.8 %, respectively), frequencies similar to what has been described elsewhere.4,9,19
In our region, few data on cancer patients have been reported.20,21 Surveillance studies conducted in the general population show a rising frequency of MDR GNR in our country. According to the SENTRY antimicrobial surveillance program,32 in Argentina 18.1 % of E. coli and 60.4 % of Klebsiella spp. isolates produce ESBL, and 8.2 % of Klebsiella spp. and 53.8 % of P. aeruginosa are meropenem-nonsusceptible. According to the RAM WHONET-Argentina,33 CPE are found in more than 250 hospitals in our country. A recent multinational observational study was conducted in seven Latin American countries including Argentina.34 Of 255 bloodstream infections, 21 % were caused by CPE producing Enterobacteriaceae, which were mostly KPC (83 %).
The variables herein described as risk factors for MDR GNR have been previously reported in other studies.9,16,35,36 That is the case of fecal colonization with MDR GNR. In a recent Italian multicenter study in SCT patients,36 colonization with resistant GNR was associated with an increased rate of infection by the same pathogen, especially carbapenem-resistant Klebsiella and MDR PSA. In other report17 CPE fecal colonization was subsequently followed by bacteremia in 45 % of neutropenic patients. This association between fecal colonization and subsequent bacteremia has also been documented for several MDR GNR, such as ESBL-E, MDR PSA and MDR Steno.9,16,35 Our study has shown that colonization or previous infection with MDR GNR is a risk factor for MDR GNR bacteremia. However, it is worth mentioning that in some participating centers rectal swabs were collected heterogeneously. This was so given the different policies in each center and the current evidence that does not recommend universal MDR colonization screening in a non-outbreak settings.5,37
Recent antibiotic exposure before the episode of bacteremia has also been described as a risk factor for MDR GNR in multiple studies,4,28,38 with third and fourth generation cephalosporins, fluoroquinolones and carbapenems being the most implicated agents.9,18,28,35
Further, recent and prolonged hospitalization has been largely reported as a risk factor for MDR GNR, mainly through horizontal transmission and secondary to exposure to nosocomial bacteria and antibiotic use. That is also the case for recent hospitalization in an intensive care unit.4,9,17,18,35,38
The presence of severe mucositis has been associated with MDR GNR bacteremia, secondary to the presence of lesions and ulcerations in the gastrointestinal mucosal barrier.39 It has been further suggested that in HSCT patients, severe mucositis represents the most important risk factor for bacteremia.39
This study describes a clinical score to predict the risk for MDR GNR bacteremia in patients with cancer and HSCT. This score consists of five (above mentioned) variables graded one point each. Using a cut-off value of two points, the score has a moderate sensitivity (66.07 %), a moderate to high specificity (77.8 %), and moderate PPV and NPV (68 % and 75.9 %, respectively). Interestingly, these results have been obtained in a local setting of high MDR GNR prevalence. Thus, in regions with a lower prevalence of MDR GNR, this score may have a higher NPV, and it would be more useful to rule out MDR GNR bacteremia. Likewise, as described in Table 3, in the cases with one or none of the proposed risk factors in an episode of bacteremia, the negative LR is 0.18 and the post-test probability of having an MDR GNR would be 11.68 %.
Similar scores have been proposed to evaluate this problem. Thus, a prospective study carried out in Italy17 with patients colonized with KPC showed that previous rectal colonization, previous intensive care unit stay, invasive abdominal procedures, and recent chemotherapy or radiotherapy were associated with KPC bacteremia, thus proposing a high NPV score. A study conducted in the United States35 proposed a decision-tree to predict the probability of ESBL producing GNR bacteremia. Predicting variables were previous infection or colonization with ESBL GNR, having a central venous catheter, age ≥ 43 years old, recent hospitalization, and previous antibiotic treatment. This model had an elevated PPV and NPV. A more recent study16 showed a clinical risk score for ESBL-E bacteremia in hospitalized patients, with a high NPV. Unlike ours, these three studies evaluated all hospitalized patients, not only cancer patients. However, around 35 %, 40 % and 20 % had an underlying malignancy, respectively.
Among patients with hematological malignancies, a retrospective study carried out in France18 evaluated the risk factors for cefepime resistance. Recent hospitalization, previous use of antibiotics and acute lymphoblastic leukemia proved to be associated with infections with cefepime resistant bacteria, thus proposing a score with these three variables. However, they included GNR and GPC resistant to cefepime. Furthermore, as France has a low prevalence of MDR GNR, its results cannot be easily extrapolated to our local epidemiology.
In our cohort, MDR GNR bacteremia was associated with less adequate EAT, despite the use of more carbapenems and colistin. Consequently, all outcomes, such as breakthrough bacteremia, 7- and 30-day mortality, were worse in the MDR GNR group. These findings have been previously described.1,2,14,28,40 Thus, for an adequate EAT, risk factors for MDR GNR should be identified.
The strengths of our study rely on its being the first prospective, multicenter study carried out in Argentina, where a large number of bacteremia episodes have been enrolled, and scarce local information is available. Risk factors were identified, and a risk score was proposed. However, Argentina currently has a high local prevalence of MDR GNR, which will probably continue to rise. Therefore, the score could not be applicable to areas with different epidemiology. Notwithstanding that, in regions with a lower MDR GNR prevalence, it could be useful to rule out MDR GNR bacteremia, using a good LR and NPV. Because the MDR GNR analyzed are a heterogeneous group and consequently, the target antibiotics may differ depending on the bacteria isolated, in patients without these risk factors piperacillin/tazobactam or cefepime may be used safely. In the cases with a high risk for MDR GNR, we strongly consider prior MDR GNR colonization to select the appropriate empirical agent. We usually suggest to start empirically with a carbapenem in this scenario, especially when there is hemodynamic instability, due to the risk for ESBL,23 and in patients that are colonized with KPC or MDR PAE we usually favor the use of ceftazidime-avibactam41 or ceftolozane-tazobactam,42 respectively, instead of using other nephrotoxic agents such as amikacin or colistin. We also understand that several other models have been developed to predict for ESBL-E,16 KPC17 or even MDR PAE bacteremia,43 and these can also prove to be useful tools in these high-risk patients. We also acknowledge that the population analyzed is heterogeneous in terms of cancer type and the presence of neutropenia. However, we intended to group these patients to explore these variables as risk factors for MDR-GNR, knowing that bloodstream infections in cancer patients are heterogeneous and may present in patients with even a minor degree of immunosuppression. As far as score variables, the data on previous colonization with MDR GNR may be underestimated because rectal swabs were collected heterogeneously (only done in 60 % of the participating institutions), not performed in 129 patients (32.7 %). Finally, in order to clearly estimate the utility of this score in the clinical practice, it must be validated in a prospective cohort. Therefore, we are currently enrolling patients in the National Registry of Bacteremia in Cancer and HSCT patients.
ConclusionAs a consequence of the rise in antimicrobial resistance, treatment of GNR bacteremia in cancer patients is a challenge. Multiple approaches must be made to minimize the impact of MDR GNR infections on cancer patients. Moreover, patient stratification using the proposed score once a patient has a GNR bacteremia could be a potential tool for clinicians, which would require prospective validation.
Authors’ contributionsAAC and FH participated in the conception and design of the study. AAC, AL, IRR, CJP, RJ, AV, AN, PC and MD participated in the acquisition of clinical and microbiology data. AAC participated in the analysis of data. AAC, AL, IRR, CJP, RJ, AV, AN, PC, MD and FH participated in the drafting of the manuscript and critical revision. All authors read and approved the final manuscript.
FundingThis work was supported by Norberto Quirno Foundation scholarship. This foundation, however, did not participate in the study design, sample collection and analysis and data interpretation, or in the decision to submit the manuscript for publication. Only the authors had full access to the study data files.
Availability of data and materialsData is available from the corresponding author upon request.
Ethics approval and consent to participatePatient participation in this study was voluntary, and a written informed consent was required. This study was approved by CEMIC Ethics Committee (Approval number 880) and the ethics committees from the different participating institutions.
Conflictsof interestThe authors declare no conflict of interests.
Other members of the ROCAS groupPablo Bonvehí, Elena Temporiti, Soledad Zárate, Jorgelina Smayevsky, Fernando Poletta (CEMIC), Pilar Vizcarra, Amadeo Esposto, Rosana Padlog (Hospital HIGA Gral. San Martín, La Plata), Laura Barcán, Graciela Greco (Hospital Italiano de Buenos Aires), Graciela Guerrini, Aníbal Calmaggi, Vanina Torres (Hospital HIGA Dr. Rodolfo Rossi, La Plata), Victoria Pinoni, Ernesto Efrom, Jorge Martínez, Marta Giovanakis (Hospital Británico de Buenos Aires), Agustina Racioppi, Carla Iglesias, Alejandra Valle (Instituto Alexander Fleming, Buenos Aires), María Luz González Ibáñez, Cristina Damiano (FUNDALEU), María Laura Chaves, Patricia Kasardyian (Hospital Municipal de Oncología Marie Curie), Martín Luck, Marcelo Bronzi, Patricia García, Mara Vallejos (Instituto de Oncología Angel H. Roffo), Juan Pablo Caeiro, Carlos Vilaró (Hospital Privado Centro Médico de Córdoba), Alejandra Corso, Fernando Pasterán (ANLIS “Dr. C. Malbrán”, Buenos Aires).
We thank the dedicated ROCAS Group participants. We thank Valeria Melia, scientific translator at CEMIC Research Unit, for English edition of the manuscript. Data from this study were previously presented, in part, at the 2017 ID Week, San Diego, CA (poster 2380, Session: 254. Transplantation - Bacterial Infections) and at the XVII Argentine Society of Infectious Diseases (SADI) Congress, Mar del Plata, 2017 (oral presentation).
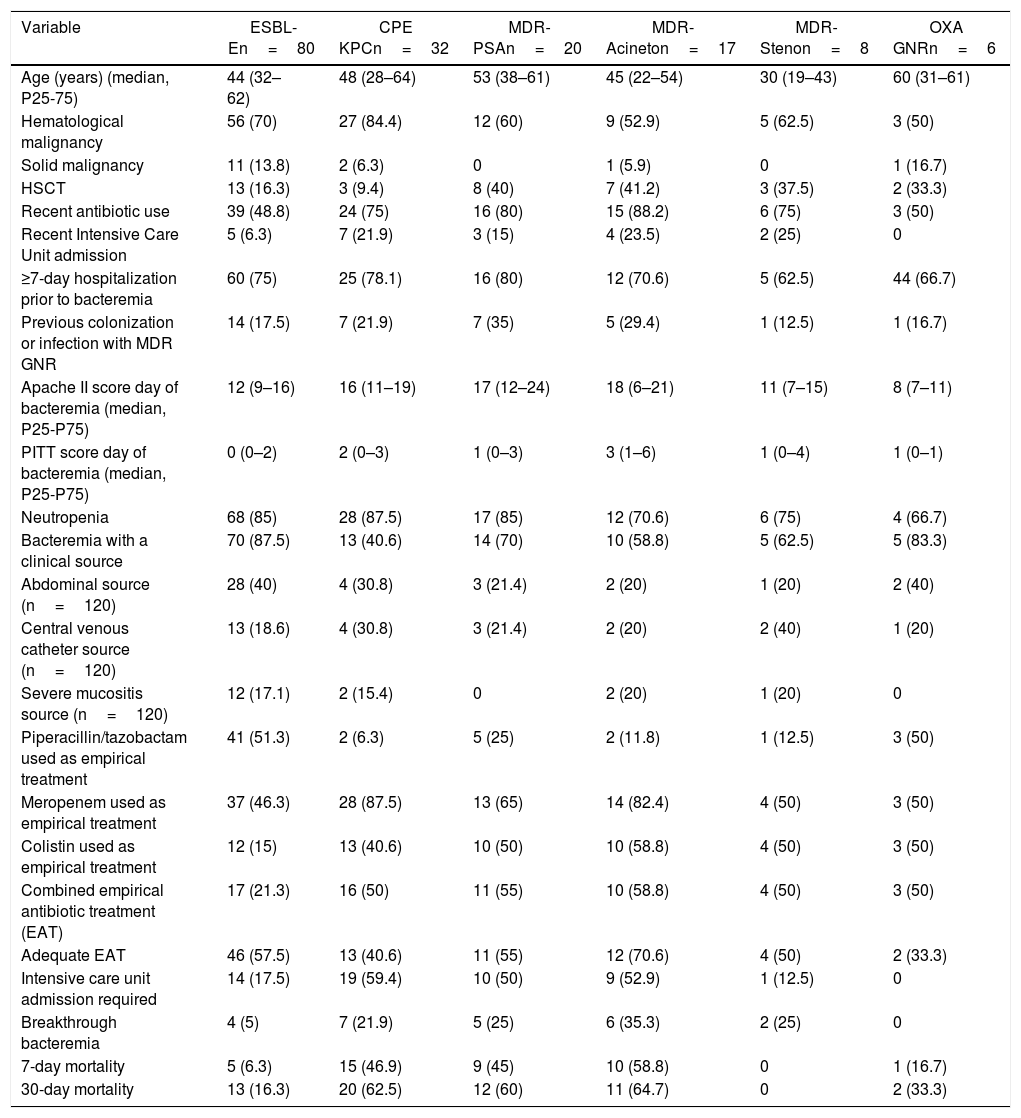
Characteristics of episodes caused by the most frequent MDR GNR (n=168).
| Variable | ESBL-En=80 | CPE KPCn=32 | MDR-PSAn=20 | MDR-Acineton=17 | MDR-Stenon=8 | OXA GNRn=6 | |
|---|---|---|---|---|---|---|---|
| Age (years) (median, P25-75) | 44 (32–62) | 48 (28–64) | 53 (38–61) | 45 (22–54) | 30 (19–43) | 60 (31–61) | |
| Hematological malignancy | 56 (70) | 27 (84.4) | 12 (60) | 9 (52.9) | 5 (62.5) | 3 (50) | |
| Solid malignancy | 11 (13.8) | 2 (6.3) | 0 | 1 (5.9) | 0 | 1 (16.7) | |
| HSCT | 13 (16.3) | 3 (9.4) | 8 (40) | 7 (41.2) | 3 (37.5) | 2 (33.3) | |
| Recent antibiotic use | 39 (48.8) | 24 (75) | 16 (80) | 15 (88.2) | 6 (75) | 3 (50) | |
| Recent Intensive Care Unit admission | 5 (6.3) | 7 (21.9) | 3 (15) | 4 (23.5) | 2 (25) | 0 | |
| ≥7-day hospitalization prior to bacteremia | 60 (75) | 25 (78.1) | 16 (80) | 12 (70.6) | 5 (62.5) | 44 (66.7) | |
| Previous colonization or infection with MDR GNR | 14 (17.5) | 7 (21.9) | 7 (35) | 5 (29.4) | 1 (12.5) | 1 (16.7) | |
| Apache II score day of bacteremia (median, P25-P75) | 12 (9–16) | 16 (11–19) | 17 (12–24) | 18 (6–21) | 11 (7–15) | 8 (7–11) | |
| PITT score day of bacteremia (median, P25-P75) | 0 (0–2) | 2 (0–3) | 1 (0–3) | 3 (1–6) | 1 (0–4) | 1 (0–1) | |
| Neutropenia | 68 (85) | 28 (87.5) | 17 (85) | 12 (70.6) | 6 (75) | 4 (66.7) | |
| Bacteremia with a clinical source | 70 (87.5) | 13 (40.6) | 14 (70) | 10 (58.8) | 5 (62.5) | 5 (83.3) | |
| Abdominal source (n=120) | 28 (40) | 4 (30.8) | 3 (21.4) | 2 (20) | 1 (20) | 2 (40) | |
| Central venous catheter source (n=120) | 13 (18.6) | 4 (30.8) | 3 (21.4) | 2 (20) | 2 (40) | 1 (20) | |
| Severe mucositis source (n=120) | 12 (17.1) | 2 (15.4) | 0 | 2 (20) | 1 (20) | 0 | |
| Piperacillin/tazobactam used as empirical treatment | 41 (51.3) | 2 (6.3) | 5 (25) | 2 (11.8) | 1 (12.5) | 3 (50) | |
| Meropenem used as empirical treatment | 37 (46.3) | 28 (87.5) | 13 (65) | 14 (82.4) | 4 (50) | 3 (50) | |
| Colistin used as empirical treatment | 12 (15) | 13 (40.6) | 10 (50) | 10 (58.8) | 4 (50) | 3 (50) | |
| Combined empirical antibiotic treatment (EAT) | 17 (21.3) | 16 (50) | 11 (55) | 10 (58.8) | 4 (50) | 3 (50) | |
| Adequate EAT | 46 (57.5) | 13 (40.6) | 11 (55) | 12 (70.6) | 4 (50) | 2 (33.3) | |
| Intensive care unit admission required | 14 (17.5) | 19 (59.4) | 10 (50) | 9 (52.9) | 1 (12.5) | 0 | |
| Breakthrough bacteremia | 4 (5) | 7 (21.9) | 5 (25) | 6 (35.3) | 2 (25) | 0 | |
| 7-day mortality | 5 (6.3) | 15 (46.9) | 9 (45) | 10 (58.8) | 0 | 1 (16.7) | |
| 30-day mortality | 13 (16.3) | 20 (62.5) | 12 (60) | 11 (64.7) | 0 | 2 (33.3) | |
Tetrachoric correlation of the variables included in the score.
| MDR GNR | ≥ 7-day hospitalization | Recent antibiotic use | Recent or previous colonization or infection | Recent Intensive Care admission | Severe Mucositis | |
|---|---|---|---|---|---|---|
| MDR GNR | 1 | |||||
| ≥7-day hospitalization | 0.3793 | 1 | ||||
| Recent antibiotic use | 0.3059 | 0.1843 | 1 | |||
| Recent or previous colonization or infection | 0.1957 | 0.0703 | 0.1963 | 1 | ||
| Recent Intensive Care admission | 0.1782 | 0.0784 | 0.1801 | 0.1337 | 1 | |
| Severe Mucositis | 0.1574 | 0.1258 | −0.0967 | −0.0912 | −0.0307 | 1 |