The diagnosis of progressive disseminated histoplasmosis is often a challenge to clinicians, especially due to the low sensitivity and long turnaround time of the classic diagnostic methods. In recent years, studies involving a variety of non-culture-based diagnostic tests have been published in the literature. We performed a systematic review by selecting studies evaluating non-culture-based diagnostic methods for progressive disseminated histoplasmosis. We searched for articles evaluating detection of antibody, antigens, as well as DNA-based diagnostic methods. A comprehensive PUBMED, Web of Science, and Cochrane Library search was performed between the years 1956 and 2016. Case reports, review articles, non-human models and series involving less than 10 patients were excluded. We found 278 articles and after initial review 18 articles were included: (12) involved antigen detection methods, (4) molecular methods, and (2) antibody detection methods. Here we demonstrate that the pursuit of new technologies is ultimately required for the early and accurate diagnosis of disseminated histoplasmosis. In particular, urinary antigen detection was the most accurate tool when compared with other diagnostic techniques.
Histoplasmosis is a fungal disease caused by the thermally dimorphic fungus Histoplasma capsulatum.1 Humans develop histoplasmosis by inhaling H. capsulatum spores from the environment, usually in the context of acid and moist nitrogen-rich soils containing excrement from poultry, bats, or birds. Even though histoplasmosis may be a self-limiting disease, disseminated infection may occur particularly in the immunocompromised host. Diagnosis of progressive disseminated histoplasmosis (PDH) has historically been made by combination of fungal culture and histopathology. However, these may require invasive medical procedures to obtain tissues, and cultures may take up to six weeks to reveal fungal growth.2,3 Moreover, in recent years a variety of non-culture-based diagnostic methods have been developed to diagnose PDH aiming for an early and more sensitive diagnosis.2 In this study we performed a systematic review of the available data on these novel technologies, summarizing their performance.
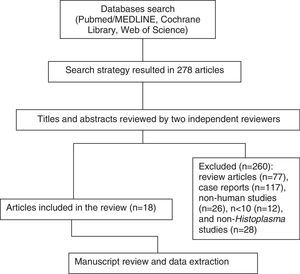
MethodsA computerized search without language restrictions was conducted in Pubmed/MEDLINE, Web of Science, and Cochrane Library, for articles published up to June 2016 combining the following terms ((DISSEMINATED HISTOPLASMOSIS) AND (ANTIBODY OR MOLECULAR OR “POLYMERASE CHAIN REACTION”[MH] OR ANTIGEN OR IMMUNODIFFUSION OR “COMPLEMENT FIXATION TESTS/METHODS”[MAJR] OR “LATEX FIXATION TESTS”[MH]) AND (DIAGNOSIS)). Only original articles dealing with non-culture-based diagnostic tests for PDH were studied. References from selected articles were also screened for review. Publications describing case reports, review articles, case series involving <10 patients, and histoplasmosis in non-humans were not included in the review (Fig. 1).
This systematic review aimed to summarize the performance of non-culture-based diagnostic methods for the detection of H. capsulatum, focusing on three distinct test groups: (i) antibody detection tests, including immunodiffusion, complement fixation and latex agglutination; (ii) antigen detection tests, including enzyme immunoassays (EIAs); and (iii) molecular methods. A total of eighteen studies were included in the review (Table 1).
Studies included in the systematic review.
| Reference | Method | n | Population studied | Main results |
|---|---|---|---|---|
| Arango-Bustamante et al., 2013 | Serology (CF and ID) | 391 | HIV and non-HIV, PDH patients | Positivity non-HIV: CF 87%, ID 95%; HIV: CF 79%, ID 92% |
| Gerber et al., 1972 | Serology (CF and LA) | 70 | Acute, chronic pulmonary and PDH, individuals without histoplasmosis | Positivity: Acute, LA: 97%, CF: 91%. Chronic, LA: 96%, CF: 91%. PDH, LA: 64%, CF: 82% |
| Gutierrez et al., 2008 | Antigen detection (MVista® EIA) | 21 | AIDS patients with PDH | Sensitivity: Panamanian patients, 95.2%; US patients, 100% |
| Cloud et al., 2007 | Antigen detection (IMMY® EIA and MVista® EIA) | 99 | Random urine samples from reference laboratory | Agreement for positive samples: 92% Negative samples: 98% |
| Scheel et al., 2009 | Antigen detection (ELISA developed by CDC) | 217 | AIDS patients from Guatemala | Sensitivity: 81%; specificity: 95% |
| Theel et al., 2013 | Antigen detection (comparison between IMMY® EIA and MVista® EIA) | 1003 | Random urine samples | Overall agreement of 97.6% |
| Connolly et. al., 2007 | Antigen detection (MVista® EIA 2nd generation) | 130 | PDH patients and controls | Sensitivity: 100%; specificity: 99% |
| Zimmerman et al., 1989 | Antigen detection (comparison between AP-ELISA, HRP-ELISA and RIA) | 19 | PDH | AP- and HRP-ELISA: 89.5% positivity; RIA: 94.7% positivity |
| Wheat et al., 1989 | Antigen detection (HPA-ELISA) | 61 | PDH, AIDS patients | Positivity, urine: 96.7%; blood: 78.7% |
| Durkin et al., 1997 | Antigen detection (comparison between RIA and EIA) | 182 | PDH patients and controls | Correlation coefficient: 0.974 |
| LeMonte et al., 2007 | Antigen detection (comparison between IMMY® EIA and MVista® EIA) | 50 | PDH patients and controls | Sensitivity for IMMY®: 44%; specificity: 84% for IMMY®, 98% for MVista® |
| Gomez et al., 1997 | Antigen detection (MAb ELISA) | 35 | PDH in AIDS and non-AIDS patients, acute and chronic histoplasmosis | Overall sensitivity 71.4%; PDH, 62.5–72.7% |
| Theel et al., 2015 | Antigen detection (IMMY® EIA with protocol modification) | 150 | Suspected PDH patients | 90% of agreement with MVista® EIA |
| Caceres et al., 2014 | Antigen detection (ELISA developed by CDC) | 106 | AIDS patients with suspected histoplasmosis | Sensitivity: 86%; specificity: 94% |
| Babady et al., 2011 | DNA detection (PCR) | 797 | Patients with suspected fungal infection | Sensitivity: 73%; specificity: 100% |
| Tang et al., 2006 | DNA detection (PCR) | 76 | Urine samples from patients with histoplasmosis and controls | Sensitivity: 7.8% |
| Maubon et al., 2007 | DNA detection (PCR) | 40 | Suspected PDH patients | Sensitivity: 100% in culture-positive |
| Scheel et al., 2014 | DNA detection (LAMP) | 16 | AIDS patients with PDH and controls | Sensitivity: 67% in antigen-positive |
AP, alkaline phosphatase; CDC, Centers for Disease Control and Prevention, Atlanta; CF, complement fixation test; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; HRP, Horseradish Peroxidase; ID, immunodiffusion test; LA, latex agglutination test; LAMP, loop-mediated isothermal amplification; MAb, monoclonal antibody; PCR, polymerase chain reaction test; PDH, progressive disseminated histoplasmosis; RIA, radioimmunoassay; US, United States of America.
Currently, two main serological tests are used for the detection of H. capsulatum antibodies: immunodiffusion and complement fixation.2 Although these methods have the advantage of being non-invasive, but enclose several limitations, including (i) marked intra-patients variation in results; (ii) long time for positive results (up to six weeks are required after exposure for antibody production); (iii) potential cross-reactivity with antibodies produced by other fungi such as Blastomyces dermatitidis.1–5
The immunodiffusion method is widely used in clinical practice and it is based on the precipitation of the anti-M and anti-H antibodies. This method is more specific than the complement fixation2,3 and presents the following strengths: (i) it is based on simple and reliable methodology; (ii) it has a low cost; (iii) specificity is close to 70–100%.2,4 Test sensitivity is unacceptably low in the immunocompromised population, particularly in individuals with AIDS.
The complement fixation method is more sensitive than the immunodiffusion test and presents variable test sensitivity, depending on the antigen phase, ranging from 72.8% in mycelial to 94.3% in yeast phase. The specificity varies between 70% and 80%; cross-reactions may occur with blastomycosis, candidosis, and paracoccidioidomycosis. Antibody titers of 1:8 and 1:16 are frequently seen in individuals with past infections or living in endemic regions and these are considered weakly positive results. Test sensitivity is reduced in the presence of hemolytic and lipemic samples.2,3
H. capsulatum may also be detected by the means of latex agglutination test. The test is based on latex connection with histoplasmin for the detection of the antibody anti-Histoplasma. Studies conducted in mid to late 1970s demonstrated that the latex test was not well-suited for diagnosis of PDH due to low sensitivity (64%) and cross-reactivity with tuberculosis, in a comparison with the gel immunodiffusion.5 Main test advantages are low cost and better specificity, as compared to the complement fixation test, despite of false-positives observed with M. tuberculosis infection.2,5
Immunological tests: enzyme immunoassays (EIAs)EIAs are based on the detection of the H. capsulatum polysaccharide antigen (HPA) on various biological materials such as urine, serum, and bronchoalveolar washing fluid. Different EIAs have been used as surrogate of PDH by reference laboratories.6–9 Two commercial EIA tests to detect H. capsulatum are currently available in the United States of America: MVista in Indianapolis, IN, and IMMY in Norman, OK. Both laboratories provide similar and robust EIA tests with minor differences in terms of test performance. However, there has been a strong debate in the literature on difference between them, which seems to be influenced by a marked commercial bias.8,10–13
In 1989, Wheat and other researchers from Miravista Diagnostics (Indianapolis, IN) developed a rapid and promising method for the detection of urinary antigens in PDH, detecting antigens in 90% of urine and 50% of blood samples in patients with the disease.14 The MVista®Histoplasma antigen test is based on a quantitative sandwich enzyme immunoassay designed for serum, plasma, urine, broncoalveolar lavage fluid and other body fluid testing.9 The manufacturer recommends the test not to be employed as the exclusive diagnostic tool in patients with histoplasmosis, since cross-reactions may occur with blastomycosis, paracoccidioidomycosis, penicilliosis, sporotrichosis, coccidioidomycosis (less frequently), and aspergillosis (rarely).9 In 2006, the test was upgraded to its second generation eliminating false positives from the previous method.15 Beside diagnosis, the test can also be used to monitor treatment response to amphotericin B.14 Although the MVista®Histoplasma antigen test has been largely studied in HIV-infected patients, the test is only performed at the MiraVista Diagnostics headquarters’ in Indiana, USA.9,11,13–15 This is the main limitation to the use of the MVista® test in other parts of the world.
The IMMY® ALPHA ELISA kit (IMMY, Norman, OK) is 2-step sandwich-type immunoenzymatic assay using polyclonal antibodies which quantitatively detects Histoplasma antigens in urine samples. It can be used in addition to other diagnostic techniques such as culture, histology, and radiology. This test was developed and validated in 2007 at the University of Utah by Cloud and colleagues.6
The main disadvantage of the IMMY test is the cross-reactivity with dimorphic fungal culture filtrate antigens of C. immitis, P. brasiliensis, and B. dermatiditis.6 Additional limitations for the IMMY test is that the test has been validated mostly in urine samples and cannot be used for other biological samples, such as serum or broncoalveolar lavage fluid.6
A study by LeMonte and other researchers of Miravista Diagnostics, comparing IMMY® and MVista®, found a lower sensitivity (44%) and specificity (84%) for IMMY®.12 However, a strong reaction to this study came in a letter from the developers of IMMY®, denouncing technical and clinical flaws.10
Theel and colleagues also compared IMMY® and Mvista® tests, showing that an overall agreement of 97.6% between the tests. Nevertheless, IMMY® showed sensitivity of only 64.5% in this study. The article points out that the MVista® could identify patients negative for the IMMY® test and both methods present cross-reactions.8
The recent news is that IMMY is about to launch an ELISA kit using monoclonal antibodies against H. capsulatum that promise to be more sensitive and specific than the current assay based on polyclonal antibodies. Hamilton and colleagues used monoclonal antibodies screening cross-reactivity to dimorphic fungi with similar antigens, as Histoplasma, Blastomyces, Sporothrix and Paracoccidioides species.16 In 2015 Theel and colleagues updated the cut-off points, optimizing the IMMY® GM ASR test (using monoclonal antibodies) and performed a comparison with the MVista EIA, detecting Histoplasma earlier.17 The lateral flow test is also under development by IMMY and may allow for a real point-of-care diagnosis for histoplasmosis, as it is available for cryptococcosis.
Considering the HIV epidemic and the increased recognition of histoplasmosis as an opportunistic disease and cause of death in endemic countries, the US Centers for Disease Control and Prevention (CDC) developed an in house enzyme-linked immunosorbent assay (ELISA) for H. capsulatum detection. The test was initially validated in a study conducted in Guatemala.7 This assay identifies specific H. capsulatum antigens using polyclonal antibodies, with 81% sensitivity and 95% specificity. Cross-reactions occurred for patients with paracoccidioidiomycosis. Similar results were obtained in another study conducted in Colombia, with 86% sensitivity and 94% specificity.18
The detection of galactomannan (GM), a polysaccharide that is mostly found in the cell wall of Aspergillus species, is largely used in clinical practice for the diagnosis of invasive aspergillosis in neutropenic patients. As in AIDS patients histoplasmosis if far more common than aspergillosis, GM detection has been suggested as a potentially helpful diagnostic test for histoplasmosis in this patient population.19–22 In a prospective study with 78 HIV-positive patients with suspected PDH, GM testing in the urine had limited sensitivity (12.5%) for the diagnosis of PDH (Hoffmann E, unpublished data).
Molecular methodsThe development of polymerase chain reaction (PCR) methodologies for H. capsulatum has been the focus of many laboratories for a more rapid and sensitive detection of such agent in tissue and body fluids. In order to achieve a higher sensitivity and specificity a number of PCR methods targeting different regions of the H. capsulatum genome were created, including conventional, nested, and real-time PCR.23–26 PCR methodology may be more sensitive and specific than immunological or serological testing, but the absence of commercially available FDA-approved methods (most PCR-based methods are homebrew technology without external validation) limit the generalization of Histoplasma PCR results.24
Based on the search and inclusion criteria, only four studies were selected when considering molecular methods.23–26 As a limitation, molecular biology methods vary in different aspects, including DNA targets, reagents, primers, probes, and platforms.
Babady et al. in 2001 developed and validated a real-time PCR assay using 797 different clinical samples (not including urine), showing sensitivity of 73% (11/15) and specificity of 100% (782/782) for H. capsulatum.23 However, when using urine samples, Tang and colleagues found a very low sensitivity (7.8%) in 51 samples proven to be antigen-positive by MVista®.26 Another study used the nested PCR in 40 different samples from patients suspected of disseminated histoplasmosis, and showed 100% sensitivity in culture-positive samples.24 The Loop-Mediated Isothermal Amplification (LAMP) is a nucleic acid amplification technique based on the helicase activity of Bst polymerase from Bacillus stearothermophilus. One of the advantages of the LAMP technique relies on the fact that the Bst polymerase is cheaper than Taq enzyme (used in PCR reactions). Also, it involves minimum handling time and material usage. Since results may be interpreted using UV light, this assay may be used in resource-limited laboratories. Although promising, LAMP presents some weakness for diagnosis of PDH: first, the method was only tested in urine samples; second, sensitivity remains low (67%); and third, the difficulty of rapidly and inexpensively extracting high-quality fungal DNA remains challenging.25
ConclusionsDespite presenting a cosmopolitan distribution, the exact frequency of histoplasmosis is still not known across the world, as only few laboratories in endemic countries are equipped, prepared and able, to correctly perform the diagnosis.
According to the Global Action Fund for Fungal Infections (GAFFI), PDH is a prevalent disease in AIDS patients (15.4cases/1000people/year) and present an overall lethality as high as 45.3%. There is a major need for implementation of a global traceability system, toward a better understanding of the disease.
Diagnosis of disseminated histoplasmosis might be possible at early stages using non-culture-based methods, reducing hospitalization costs, and increasing patients’ survival. Several diagnostic methods have been developed in this regard, in particular immunologic methods for the detection of Histoplasma antigens in urine samples, and real-time PCR. Even though these infections are hyperendemic in the American continent, there is an urgent need to provide countries with newer, faster, and sensitive techniques, in order to improve patients’ outcomes.
Conflicts of interestD.R.F. has received research support from United Medical and Bagó Laboratories (now TEVA). He has been a member of the mycology advisory panel of United Medical and he has received travel grants from Gilead, Astellas, Pfizer and United Medical. He has given payed lectures for United Medical and Pfizer.
A.C.P. has consulted, received research grants, being a speaker and/or received travel grants for Gilead, United Medical, Pfizer, MSD, Schering-Plough, Astellas, Bagó, Tecnofarma, Biometrix and Myconostica.
Other authors declare no conflicts of interest.