Hepatitis E virus (HEV) infection in patients with pre-existing liver disease has shown high morbidity and lethality. The consequences of HEV superinfection in patients with chronic hepatitis C virus (HCV) infection are not fully understood. This study aimed to evaluate the association between the presence of anti-HEV antibodies, liver cirrhosis, and insulin resistance.
MethodsA total of 618 patients chronically infected with HCV were included from three reference centers for viral hepatitis in São Paulo, Brazil. Presence of anti-HEV IgG was assessed by enzyme-linked immunosorbent assay (WANTAI HEV-IgG ELISA).
ResultsThe seroprevalence of anti-HEV in patients with cirrhosis was significantly higher than in patients without cirrhosis (13.2% vs 8%, OR=1.74, p=0.04). Seropositivity for anti-HEV, adjusted for sex, age, and HCV genotype showed an association trend with hepatic cirrhosis (aOR=1.75, p=0.059). Presence of HEV antibodies, adjusted for age, body mass index and cirrhosis, was shown to be independently associated with insulin resistance (aOR: 4.39; p=0.045).
ConclusionPatients with chronic hepatitis C are under risk of hepatitis E virus superinfection in Brazil. The trend toward association between cirrhosis and previous HEV infection suggests that it may accelerate liver fibrosis in patients with chronic hepatitis C. In addition, previous infection by HEV is independently associated with insulin resistance in the studied population, which may be an extra-hepatic manifestation of hepatitis E that persists after resolution of the active infection, and may contribute to fibrosis progression.
Hepatitis E virus (HEV), a small non-enveloped RNA-virus of the Hepeviridae family, is responsible for around 20 million infections per year worldwide, with more than three million symptomatic infections, and 44,000 deaths in 2015, according to the last Global Hepatitis Report of the World Health Organization (WHO).1
To date, five main HEV genotypes capable of infecting humans have been described. Genotypes 1 and 2 are restricted to human beings, occur in large epidemics, mainly in underdeveloped countries, and have enteric transmission, similarly to hepatitis A virus (HAV). Genotypes 3 and 4 are described sporadically in developed countries, have endemic circulation, and zoonotic reservoir.2 Genotype 7, also zoonotic, was described in a patient who regularly consumed camel meat and milk.3
WHO estimates that in 2015, 71 million people, representing about 1% of the world population, were living with hepatitis C virus (HCV) infection.1 Previous studies have demonstrated high morbidity and mortality rates due to HEV infection in patients with underlying chronic liver disease4 and after superinfection by other hepatotropic viruses in patients with chronic HCV.5,6
Furthermore, several studies have shown association of metabolic syndrome and insulin resistance with hepatic cirrhosis and infection by other hepatotropic viruses, particularly HCV. The relationship between HEV infection and insulin resistance is unknown.7,8
The consequences of HEV infection in patients with long-term chronic HCV infection are not fully understood. This group of patients may be susceptible to superinfection for decades, since active immunization for HEV is not available in most countries, as it is for hepatitis A virus (HAV) and hepatitis B virus (HBV).
The main objective of this study was to evaluate the association between presence of anti-HEV antibodies and hepatic cirrhosis in patients with chronic hepatitis C. As a secondary objective, the association of insulin resistance and previous HEV infection was also assessed.
Materials and methodsStudy designAs an extension of a previous cross-sectional study on HEV seroprevalence in Brazil,9 we investigated the association between anti-HEV IgG positivity, liver cirrhosis, and insulin resistance in patients with chronic HCV.
Between October 2015 and December 2016, patients were recruited from three reference outpatient clinics for viral hepatitis in the state of São Paulo, Brazil: Federal University of São Paulo, Euryclides Jesus Zerbini Transplantation Hospital, and Institute of Vaccination and Infectology of Piracicaba.
Patients of both sexes, older than 18 years, and with chronic HCV infection confirmed by RT-PCR RNA were included.
Anti-HEV antibody detectionPresence of anti-HEV IgG was assessed by enzyme-linked immunosorbent assay (WANTAI HEV-IgG ELISA), strictly according to the manufacturer's recommendations. Patients positive for HEV IgG were further tested for the presence of HEV IgM antibodies using a specific kit from the same manufacturer.
Data collectionThe following independent variables possibly associated with the dependent variable hepatic cirrhosis were evaluated: sex, age, body mass index (BMI), alcohol use, HIV infection, serological profile of HBV, HCV genotype, previous treatment for HCV, and insulin resistance.
To assess the relationship with insulin resistance, the following independent variables were evaluated: anti-HEV IgG, sex, age, BMI, cirrhosis, HCV genotype, HIV infection, and serologic profile of HBV.
Liver cirrhosis was characterized by liver biopsy with F4 score on the Metavir scale or transient hepatic elastography (Fibroscan®) with a result ≥12.5kPa10 or AST to Platelet Ratio Index (APRI) ≥2.0.11 Hepatic cirrhosis was considered absent in case of a liver biopsy with score ≤3 on the Metavir scale or not done, a Fibroscan with result <12.5kPa or not done, APRI <0.5, and Fibrosis-4 (FIB-4) score <1.45.12
All blood samples were collected in the morning with the orientation of fasting of 8–12h. The samples for glycemia and insulinemia were not frozen and were processed in the same day of collection. Insulin resistance was considered when the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) score was ≥2.7 or fasting glycemia ≥126mg/dL.13
The Alcohol Use Disorders Identification Test (AUDIT-C), a validated screening tool, was used to identify patients who binge on alcohol or with alcohol dependence. Scores higher than two points for males or more than one point for females were considered indicative of harmful alcohol use.14,15
Statistical analysisAssuming a prevalence of anti-HEV IgG of 8% in the non-cirrhotic group and 15% in the cirrhotic group, the calculated sample size was 252 patients per group, with an 80% power and significance level of 5%.
Continuous variables were summarized using means and standard deviations whereas categorical measures were summarized using absolute counts and percentages. For univariate analyses, Student's t-test was used to compare means, and Chi-square test or Fisher's exact test were used to compare proportions, as appropriate.
All associations with p-value <0.20 at univariate analyses were subsequently included into multiple logistic regression models. The selected continuous variables were categorized into dichotomous variables according to the cutoff point that yielded the highest area under the curve in ROC curve analysis. The final model was obtained using a backward strategy, excluding variables with adjusted p-value ≥0.05. Statistically significant independent associations were considered for variables with p<0.05 in the final multiple logistic regression model.
All analyses were performed using program R, version 3.3.2 (The R Foundation for Statistical Computing).
Ethical aspectsThe study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the Institutional Ethics Committee of the Federal University of Sao Paulo. A written informed consent was obtained from each patient included.
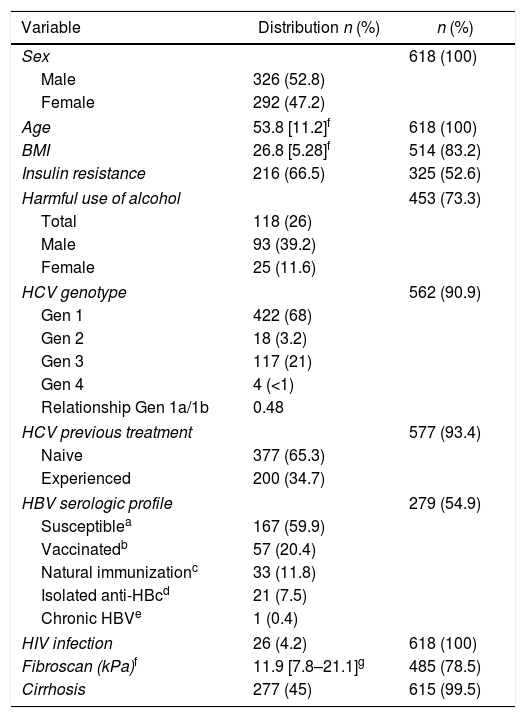
ResultsA total of 618 patients with chronic HCV infection were included. Table 1 summarizes the distribution of the variables evaluated. Fifty-three percent were male, mean age 53.8 years, ranging from 22 to 86 years. Weight and height for calculating BMI were available for 514 (83.2%) of the sample, of those 302 (58.8%) of the cases were overweight (BMI>25kg/m2). Insulin resistance was determined for 325 (52.6%) patients and present in 216 (66.5%). Fasting glycemia ≥126mg/dL was observed in 12.6% of the cases, and only two of them presented HOMA-IR <2.7. The AUDIT-C questionnaire was applied to 451 (73.3%) of the sample, of those, 117 (26%) had scores indicating harmful use of alcohol.
Clinical characteristics of the HCV infected patients.
| Variable | Distribution n (%) | n (%) |
|---|---|---|
| Sex | 618 (100) | |
| Male | 326 (52.8) | |
| Female | 292 (47.2) | |
| Age | 53.8 [11.2]f | 618 (100) |
| BMI | 26.8 [5.28]f | 514 (83.2) |
| Insulin resistance | 216 (66.5) | 325 (52.6) |
| Harmful use of alcohol | 453 (73.3) | |
| Total | 118 (26) | |
| Male | 93 (39.2) | |
| Female | 25 (11.6) | |
| HCV genotype | 562 (90.9) | |
| Gen 1 | 422 (68) | |
| Gen 2 | 18 (3.2) | |
| Gen 3 | 117 (21) | |
| Gen 4 | 4 (<1) | |
| Relationship Gen 1a/1b | 0.48 | |
| HCV previous treatment | 577 (93.4) | |
| Naive | 377 (65.3) | |
| Experienced | 200 (34.7) | |
| HBV serologic profile | 279 (54.9) | |
| Susceptiblea | 167 (59.9) | |
| Vaccinatedb | 57 (20.4) | |
| Natural immunizationc | 33 (11.8) | |
| Isolated anti-HBcd | 21 (7.5) | |
| Chronic HBVe | 1 (0.4) | |
| HIV infection | 26 (4.2) | 618 (100) |
| Fibroscan (kPa)f | 11.9 [7.8–21.1]g | 485 (78.5) |
| Cirrhosis | 277 (45) | 615 (99.5) |
HCV genotype 1 was more frequently observed (78.3%), followed by genotype 3 (21.7%). Most of the patients were HCV treatment naive (65%). Serology for HBV was available for 54.9% of the sample and 19.7% of the cases tested showed evidence of previous infection by the virus (anti-HBc positive). The sample also presented 26 (4.2%) cases of HIV infection, five hemophiliacs and two renal transplant patients.
Hepatic cirrhosis was observed in 277 (45%) cases. The diagnosis of cirrhosis was based on Fibroscan® in 83.5% of the sample studied; 39 (14%) cases were diagnosed by liver biopsy or APRI ≥2 and clinical judgment was used in two cases to characterize liver cirrhosis. In three cases, subsidiary exams and clinical evaluation were not sufficient to confirm or rule out liver cirrhosis.
Sixty-three cases were anti-HEV IgG reactive, seroprevalence of 10.2% (95% CI 8.0%–12.8%). Three (0.5%) cases had indeterminate results and were considered non-reactive for hypothesis testing. Two transplant patients, both anti-HEV IgG non-reactive, were excluded from statistical analyses. Anti-HEV IgM was screened in all reactive and undetermined cases for IgG and all turned out non-reactive.
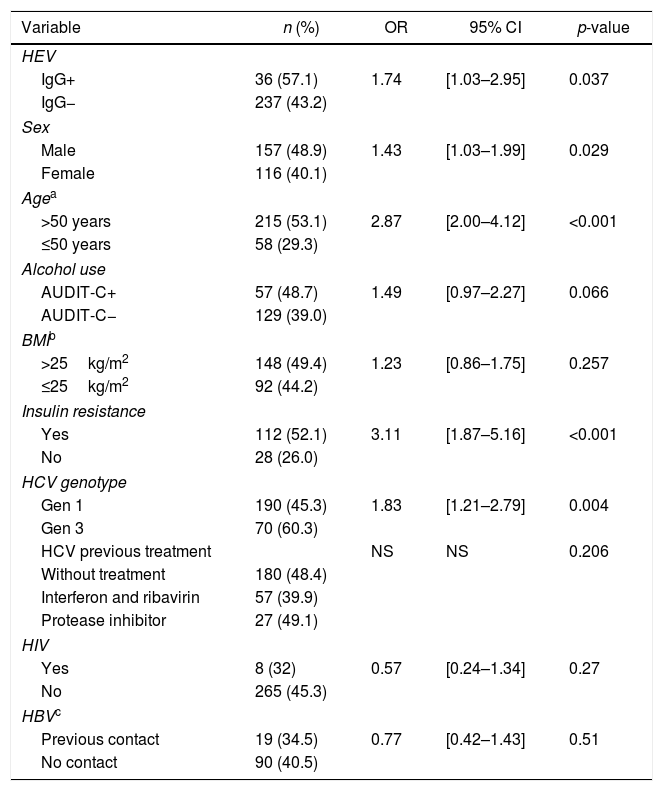
The anti-HEV positivity rate in patients with cirrhosis was significantly higher (13.2%) than in patients without cirrhosis (8%), p=0.04. Univariate analyses of variables associated with liver cirrhosis are summarized in Table 2, with significant association seen for previous HEV infection, sex, age, HCV genotype, and insulin resistance. Harmful consumption of alcohol had a borderline significance (p=0.066) and was also included in the subsequent multivariate model.
Results of univariate analyses for hepatic cirrhosis.
| Variable | n (%) | OR | 95% CI | p-value |
|---|---|---|---|---|
| HEV | ||||
| IgG+ | 36 (57.1) | 1.74 | [1.03–2.95] | 0.037 |
| IgG− | 237 (43.2) | |||
| Sex | ||||
| Male | 157 (48.9) | 1.43 | [1.03–1.99] | 0.029 |
| Female | 116 (40.1) | |||
| Agea | ||||
| >50 years | 215 (53.1) | 2.87 | [2.00–4.12] | <0.001 |
| ≤50 years | 58 (29.3) | |||
| Alcohol use | ||||
| AUDIT-C+ | 57 (48.7) | 1.49 | [0.97–2.27] | 0.066 |
| AUDIT-C− | 129 (39.0) | |||
| BMIb | ||||
| >25kg/m2 | 148 (49.4) | 1.23 | [0.86–1.75] | 0.257 |
| ≤25kg/m2 | 92 (44.2) | |||
| Insulin resistance | ||||
| Yes | 112 (52.1) | 3.11 | [1.87–5.16] | <0.001 |
| No | 28 (26.0) | |||
| HCV genotype | ||||
| Gen 1 | 190 (45.3) | 1.83 | [1.21–2.79] | 0.004 |
| Gen 3 | 70 (60.3) | |||
| HCV previous treatment | NS | NS | 0.206 | |
| Without treatment | 180 (48.4) | |||
| Interferon and ribavirin | 57 (39.9) | |||
| Protease inhibitor | 27 (49.1) | |||
| HIV | ||||
| Yes | 8 (32) | 0.57 | [0.24–1.34] | 0.27 |
| No | 265 (45.3) | |||
| HBVc | ||||
| Previous contact | 19 (34.5) | 0.77 | [0.42–1.43] | 0.51 |
| No contact | 90 (40.5) | |||
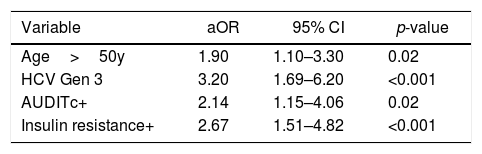
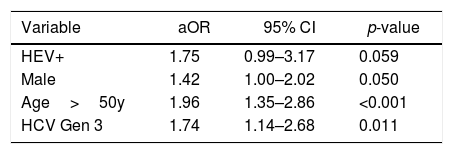
ROC curve analysis for age identified 50 years as the cutoff that best distinguished cirrhotic from non-cirrhotic. The result of the final logistic regression model is summarized in Table 3. The independent variables age, HCV genotype, harmful alcohol use, and insulin resistance were found to be independently associated with hepatic cirrhosis. On the other hand, the variables anti-HEV (aOR=0.54; 95% CI 0.19–1.50; p=0.243) and sex (aOR 1.28; 95% CI 0.74–2.22; p=0.377) lost significance. A total 346 cases were not accounted for in the multivariate analysis due to missing data, resulting in a significant loss of power. The results of a reduced multivariate model, not considering the variables insulin resistance and harmful alcohol use, are shown in Table 4. In such model, in which only 78 cases were not accounted for due to missing data, presence of anti-HEV resulted in a p-value close 0.05 [adjusted OR (aOR)=1.75; 95% CI=0.99–3.17; p=0.059].
Alternative multiple logistic regression model for hepatic cirrhosis.
| Variable | aOR | 95% CI | p-value |
|---|---|---|---|
| HEV+ | 1.75 | 0.99–3.17 | 0.059 |
| Male | 1.42 | 1.00–2.02 | 0.050 |
| Age>50y | 1.96 | 1.35–2.86 | <0.001 |
| HCV Gen 3 | 1.74 | 1.14–2.68 | 0.011 |
78 (13%) cases not accounted for due? to missing data. Independent variables AUDITc and Insulin Resistance are not considered.
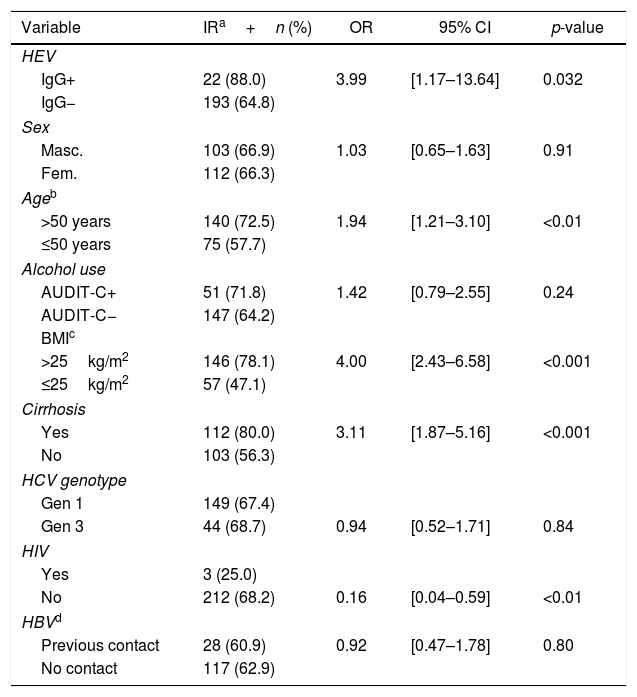
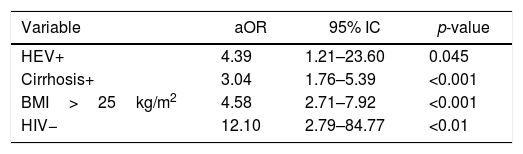
Anti-HEV positivity was significantly higher (10.2%) in patients with insulin resistance than in patients without (2.8%) [OR=3.99; 95% CI=1.17–13.64; p=0.032]. Univariate analyses of variables associated with insulin resistance are summarized in Table 5, with significant association seen for anti-HEV, age, BMI, cirrhosis, and HIV infection. ROC curve analysis for age and BMI identified 50 years and 25kg/m2, respectively, as the cutoffs that best distinguished presence of insulin resistance from absence of insulin resistance. Results of the final multiple logistic regression model are shown in Table 6. The variables anti-HEV, cirrhosis, BMI, and HIV infection were independently associated with insulin resistance. Age lost significance in the initial model (aOR=1.47; 95% CI=0.84–2.57; p=0.174).
Results of univariate analyses for insulin resistance.
| Variable | IRa+n (%) | OR | 95% CI | p-value |
|---|---|---|---|---|
| HEV | ||||
| IgG+ | 22 (88.0) | 3.99 | [1.17–13.64] | 0.032 |
| IgG− | 193 (64.8) | |||
| Sex | ||||
| Masc. | 103 (66.9) | 1.03 | [0.65–1.63] | 0.91 |
| Fem. | 112 (66.3) | |||
| Ageb | ||||
| >50 years | 140 (72.5) | 1.94 | [1.21–3.10] | <0.01 |
| ≤50 years | 75 (57.7) | |||
| Alcohol use | ||||
| AUDIT-C+ | 51 (71.8) | 1.42 | [0.79–2.55] | 0.24 |
| AUDIT-C− | 147 (64.2) | |||
| BMIc | ||||
| >25kg/m2 | 146 (78.1) | 4.00 | [2.43–6.58] | <0.001 |
| ≤25kg/m2 | 57 (47.1) | |||
| Cirrhosis | ||||
| Yes | 112 (80.0) | 3.11 | [1.87–5.16] | <0.001 |
| No | 103 (56.3) | |||
| HCV genotype | ||||
| Gen 1 | 149 (67.4) | |||
| Gen 3 | 44 (68.7) | 0.94 | [0.52–1.71] | 0.84 |
| HIV | ||||
| Yes | 3 (25.0) | |||
| No | 212 (68.2) | 0.16 | [0.04–0.59] | <0.01 |
| HBVd | ||||
| Previous contact | 28 (60.9) | 0.92 | [0.47–1.78] | 0.80 |
| No contact | 117 (62.9) | |||
This work is an extension of our previous study on HEV seroprevalence in Brazil, which found anti-HEV IgG prevalence of 10.2% (95% CI 8.0–12.8%) among patients with chronic HCV infection.9 Older age and prior contact with pigs were independently associated with Anti-HEV IgG positivity.9 In the present study, the seroprevalence in cirrhotic patients was significantly higher than in non-cirrhotic (13.2% vs 8%, OR=1.74, p=0.04). This study also demonstrated an independent association between reactive anti-HEV and insulin resistance after adjusting for age, BMI, and cirrhosis (aOR: 4.39; p=0.045).
Untreated chronic HCV infection can persist indefinitely, usually for decades, and the progression of liver fibrosis to hepatic cirrhosis is extremely variable, influenced by factors related to host, virus, and environment.16 The median progression time of the Metavir score is estimated at 0.133 per year.17 Considering this estimate, the expected median time to develop liver cirrhosis would be around 30 years, and up to one third of patients would not have cirrhosis after 50 years of infection. Some factors are associated with accelerated progression of hepatic fibrosis during chronic HCV infection, namely male sex, age over 40, alcohol use, immunosuppression (such as HIV infection), HBV coinfection, insulin resistance, and HCV genotype 3.18–21 Consistently with previous reports, the present study revealed higher rates of hepatic cirrhosis in presence of the following variables: age over 50 years (OR=2.87; 95% CI 2.00–4.14; p<0.001), male sex (OR=1.43; 95% CI 1.03–1.99; p=0.029), insulin resistance (OR=3.11; 95% CI 1.87–5.16; p<0.001), and HCV genotype 3 (OR=1.83; 95% CI 1.21–2.79; p=0.004). This study also detected a higher prevalence of cirrhosis in patients (13.2% vs 8.0%) with reactive anti-HEV IgG (OR=1.74; p=0.034), in line with what has been demonstrated for other hepatotropic viruses in previous studies.22,23
After multivariate analyses, the following variables were associated with liver cirrhosis: age, alcohol use, insulin resistance, and HCV genotype 3. Anti-HEV has not remained in the final model likely because 56% (346/618) of the cases were not accounted for in the multivariate analysis, as two of the participating centers had collected information on insulin resistance and harmful alcohol use. The final model included only 267 cases, of which 124 (46%) were cirrhotic and 143 (54%) were not cirrhotic, and only 20 cases were anti-HEV IgG positive. A recalculation of the type II error, found a significant reduction of statistical power to 27% (considering alfa=0.05 and seroprevalences of 13% vs 8% in patients with and without cirrhosis, respectively). The results of a reduced logistic regression model, excluding the independent variables of insulin resistance and harmful alcohol use, are shown in Table 4. Such reduced model, with a loss of only 78 cases (13% of the sample size) due to missing data, resulted in a clear trend toward association between anti-HEV and hepatic cirrhosis (aOR=1.75; 95% CI=0.99–3.17; p=0.059).
Few studies have assessed the impact of previous HEV infection in patients with chronic HCV infection. Mellgren et al.22 detected a significant difference of HEV seroprevalence in patients with HCV chronic infection when comparing groups with advanced hepatic fibrosis (38%) versus no significant fibrosis (24%), OR=1.97 and p=0.029. Differently from the present study, significant fibrosis was considered when one of the following criteria was present: Fibroscan ≥7kPa, liver biopsy with Metavir ≥F2 or APRI ≥0.8 in the absence of the other two criteria. The difference found was not supported by multivariate analysis due to the strong influence of the age variable. Possibly, the absence of detection of the difference is explained by the small power of the study, with only 204 cases. On the other hand, Kyvernitakis et al., when evaluating 115 patients with chronic HCV infection, found an independent association between HEV IgG seropositivity and liver cirrhosis (aOR=4.1; p=0.032).23
In analogy, a case–control study24 in patients with chronic HBV infection demonstrated greater HEV seroprevalence in patients with cirrhosis when compared to patients without cirrhosis. Among the cirrhotic patients, there was also a higher seroprevalence in the Child-Pugh-Turcotte B/C score group when compared to the A score group. It was not possible to evaluate whether the staging of hepatic cirrhosis was associated with HEV in our study due to the low number of patients with B/C scores (less than 10% of the sample). Furthermore, previous studies have also demonstrated a high risk of poor outcome of HAV and HBV superinfection in patients with chronic HCV or liver diseases.5,6 Thus, the indication of immunization for HAV and HBV in such situations is a consensus when serological profile is susceptible.
Similarly, high lethality of HEV genotype 1 infection was demonstrated in cirrhotic patients (70% in 12 months).4 However, the routine serological investigation of HEV and specific prophylactic measures are not recommended by the current management guidelines for chronic HCV infection. An HEV vaccine is licensed for use only in China since 2011. A randomized placebo-controlled phase 3 study involving 112,604 subjects evaluated the effectiveness of the recombinant HEV vaccine (Hecolin®) in a three-dose (0, 1 and 6 months) regimen with a 4.5-year follow-up.25 The effectiveness was found to be 86.8%, with adverse event rates similar to the placebo group. Another vaccine, developed by the GlaxoSmithKline laboratory, with a proven efficacy of more than 95% after the third dose, was discontinued by a decision of the laboratory in 2007. Such a decision was made despite recent evidence demonstrating widespread circulation of HEV also in the United States of America.26
After exploratory analyses of the data collected for the present study had found much higher seroprevalence of HEV among patients with insulin resistance (10.2% vs 2.8%; OR=3.47; p=0.06), we decided to extend the scope of the study performing a multivariate analysis including all variables that could also be related with the dependent variable insulin resistance. The variables independently associated with insulin resistance were: previous HEV (aOR=4.39; p=0.045), cirrhosis (aOR=3.04; p<0.001), overweight (aOR=4.58; p<0.001), and absence of HIV (aOR=12.1; p<0.01). This study also found a higher proportion of patients with fasting glycemia ≥126mg/dL in patients anti-HEV positive (20.5% versus 11.9%, p=0.130), shown in Table 7.
Several studies have reported association between hepatropic viruses and insulin resistance and metabolic syndrome, especially in HCV infection, which is interpreted by some authors, as an extra-hepatic manifestation of the disease.8,20 The mechanisms by which the HCV causes insulin resistance is not entirely understood, but certainly has multiple direct and indirect physiopathological ways, and insulin resistance itself can contribute to fibrosis progression, which may also worsen metabolic syndrome, resulting in a vicious circle. The association between HEV and insulin resistance had not been reported before, although evidence from other hepatotropic viruses suggests by analogy and plausibility that HEV can also lead to insulin resistance and metabolic syndrome. Further studies are needed to explore the association found by the present study in patients with and without underlying liver disease.
The strong association found here between absence of HIV infection and presence of insulin resistance may from result selection bias, taking into account that only 26 patients living with HIV were included and most of them had CD4 cell count greater than 500cells/mm3 and undetectable viremia, reflecting high adherence to clinical treatment. The time of this study coincided with the availability of new drugs for HCV treatment in Brazil. Thus, the participating centers had a predominant flux of patients with indications for treatment at that time in Brazil (significant liver fibrosis and HIV infection). So, the sample of the present study may not be representative of all the patients living with HCV infection.
This study presents some limitations: (1) the cross-sectional design of the study does not allow the conclusion that HEV infection occurred after HCV infection. However, considering the fact that the majority of patients assessed had been infected with HCV more than two decades in the past, and previous studies have demonstrated significantly higher seroprevalence of HEV-3 after 50 years of age, we can infer a higher probability of HEV superinfection at some point during chronic HCV infection; (2) multivariate analysis showed a substantial loss of statistical power for detecting possible associations due to incomplete data; (3) in the alternative multiple logistic regression model for cirrhosis (Table 4), age was also tested as continuous variable and the result was a p-value of 0.106 for HEV. In the final multiple logistic regression model for insulin resistance, using BMI and age as continuous variables, the result found was an adjusted p-value of 0.0886 for HEV. The different result observed in this method may be explained by a non-linear interaction between age and anti-HEV prevalence. Thus, it was decided to use a cutoff point to categorize age; (4) the present study investigated only previous infection by the HEV. The impact of recent or acute infection was not assessed; and (5) the study did not assess previous diagnosis of diabetes mellitus. Probably some diabetic patients compensated by medications were classified as without insulin resistance.
ConclusionIn this sample of patients with chronic HCV infection, there was a significantly higher seroprevalence of HEV in patients with hepatic cirrhosis compared to those without (13.2% vs 8%, OR=1.74, p=0.04). This study found a trend of association (aOR=1.75; 95% CI=0.99–3.17; p=0.059) between previous HEV infection and cirrhosis after adjustment for sex, age, and HCV genotype.
In addition, it was found an independent association between HEV previous infection and insulin resistance (aOR: 4.39; p=0.045). To the best of our knowledge, this is the first report suggesting a link between HEV and insulin resistance, which may be an extra-hepatic manifestation that persists after the resolution of HEV infection, and contributes to fibrosis progression in patients with underlying liver diseases. Further studies are warranted to confirm this early finding and to evaluate if this association is also present in non-HCV-infected individuals. The results of the present study support the assessment of HEV serological profile in patients with chronic HCV infection, as already done for HAV and HBV. Prophylactic measures should be implemented in those found susceptible, such as good hygiene practices, avoiding consumption of undercooked pork and shellfish, and active immunization, if available.
Authors contributions- •
Guilherme Bricks: main author; study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis; fund raising.
- •
Jorge Figueiredo Senise: acquisition of data; critical review of the manuscript for intellectual content.
- •
Henrique Pott-Junior, Giuliano Grandi, Dimas Carnaúba Junior and Hamilton Antonio Bonilha de Moraes: data acquisition.
- •
Celso Francisco Hernandes Granato: laboratory support, critical review of the manuscript for intellectual content.
- •
Adauto Castelo: study supervision, concept and design; data acquisition; analysis and interpretation of data; statistical analysis; fund raising; critical review of the manuscript for intellectual content.
This study was supported by the National Council for the Improvement of Higher Education (CAPES). The funding source had no involvement in the study design, conduction and writing.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by the National Council for the Improvement of Higher Education (CAPES).