Recent studies showed a high frequency of low bone mineral density (BMD) in HIV-infected patients and no reports have been issued in Turkey. Our aim was to evaluate BMD and risk factors for osteopenia/osteoporosis in HIV-infected patients that attended an outpatient clinic in Istanbul, Turkey.
MethodIn order to determine the prevalence of BMD, 126 HIV-infected patients had been studied with dual energy X-ray absorptiometry (DEXA). The association between BMD and age, gender, body mass index (BMI), habits, 25(OH)vitamin D, HIV RNA, CD4 lymphocyte nadir, using and duration of highly active antiretroviral treatment (HAART) were investigated by using multivariate analysis.
ResultsMedian age was 40.1 years (range, 20–70); 84% were male; 35.7% patients had AIDS, 63.5% were treated with HAART. Osteopenia and osteoporosis were diagnosed in 53.9% and 23.8%, respectively. Mean plasma HIV RNA was 5.2 (SD 1.0) log10 copies/mL and CD4 lymphocyte nadir was 313.8 (SD 226.2)/mm3. Factors associated with bone loss were high viral load (p=0.034), using (p=0.033) and duration of HAART (p=0.008). No correlation had been seen between sex and osteopenia/osteoporosis (p=0.794). However, males showed higher rates of osteoporosis than females (p=0.042).
ConclusionsOur results show a very high prevalence of bone mass reduction in Turkish HIV-infected patients. This study supports the importance of both HIV and antiretroviral therapy in low BMD.
HIV infected patients are now living longer with improved quality of life, but have increased risk of chronic diseases such as osteoporosis. The high prevalence of bone demineralization among younger and older HIV infected patients has been described in multiple studies and loss of bone mineral density (BMD) is associated with increased rates of bone fractures.1,2 The most common fractures are those of the vertebrae, hip and wrist. Management of hip fracture almost always require major surgical intervention and mortality rates that can be as high as 30% in the first year alone.3 Mortality has also increased following vertebral fractures, which cause significant complications including back pain, height loss and kyphosis. So, the early identification and treatment of osteopenia/osteoporosis is critical to protect the future health of HIV/AIDS patients.
There are general effects of HIV infection that can be risk factors for low BMD. These include low body mass index (BMI), physical inactivity, malabsorbtion (especially calcium), hypogonadism, vitamin D deficiency, smoking, opiat and alcohol abuse. In addition to potential causes of osteopenia/osteoporosis the literature focuses on two causative entities in HIV infection: the disease itself and its treatment.
It is well known that BMD values show differences between populations. Prevalence and risk factors of osteopenia/osteoporosis in Turkish HIV-infected population have not been reported in the literature. Thus, in this study, our aim was to evaluate the BMD and risk factors about osteopenia/osteoporosis among 126 HIV/AIDS patients who attended our outpatient clinic between June 2010 and May 2011, in Istanbul, Turkey (latitude 41°N).
Patients with known factors of osteopenia/osteoporosis (hyperthyroidism, hyperparathyroidism, hypogonadism, diabetes mellitus, use of corticosteroids and menopause) had been excluded. None of them had received calcium and vitamin D supplement previously. We analyzed the relation between BMD and age, gender, BMI, smoking (package/year), alcoholism (three or more drinks/day), illicit drug use (months), antiretroviral treatment and duration (months), CD4 cell count nadir (/mm3), HIV viral load (copy/mL), blood calcium, phosphorus, iPTH, and 25(OH)vitamin D (nmol/mL) determination. Data were collected retrospectively from standardized HIV forms filled in at admission.
BMD was measured in the posterior–anterior projection at the lumbar spine (L2–L4), at the left hip (femur neck, trochanter and Wards region) and of total body by DEXA (Norland, a CooperSurgical Company of Fort Atkinson, WI). DEXA was calibrated daily. All measurements were done by the same qualified technician. DEXA reported with T and Z-scores (T-score represents a SD of BMD mean of the population at age 30, Z-scores represents a SD of BMD mean of the same age and sex group) was performed in 126 HIV-infected patients. The patients were diagnosed as normal, osteopenic (T-score between −2.5 and −1) and osteoporotic (T-score less than −2.5) by DEXA measurements and according to World Health Organization criteria.
Total plasma samples 25(OH)D was measured by radioimmunoassay (Diasorin). The 25(OH)D levels were deemed deficient when <25nmol/mL, insufficient when 25–50nmol, sufficient when >50nmol. BMI<18.5 were considered as underweight, 18.5–24.9 as normal weight, 25–29.9 as overweight, and 30≥ as obese. CD4 cell count was determined by standard flow cytometry (FACScalibur, Becton Dickinson). Viral load was measured with the Cobas Amplicor HIV-1 (Roche). Biochemical test levels were determined by automated standard laboratory techniques.
All analyses were performed by using SPSS version 13 software. Data were described using mean±standard deviation (SD) (or median and range) and when indicated, as an absolute number and percentage. Chi-square test was carried out for categorical data and Mann–Whitney-U test was used for continuous data. A p-value less than 0.05 was considered significant. Pearson correlation was used for continuous data for investigating correlation. For this retrospective study, there was no need for ethical approval because all laboratory tests carried out were part of the routine management of HIV-infected patients.
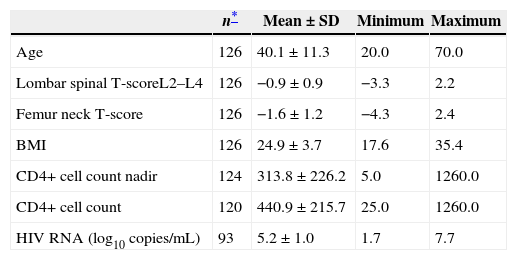
Table 1 shows analysis of the BMD, lombar spinal BMD, femur neck BMD, CD4+ cell count, CD4+ cell count nadir and HIV RNA level (log10copies/mL) of 126 cases. Of these cases, 106 (84.1%) were male, 20 (15.9%) were female. Out of the total, 45 (35.7%) had AIDS, 80 (63.5%) were treated with HAART.
Profiles of studied HIV/AIDS patients.
| n* | Mean±SD | Minimum | Maximum | |
|---|---|---|---|---|
| Age | 126 | 40.1±11.3 | 20.0 | 70.0 |
| Lombar spinal T-scoreL2–L4 | 126 | −0.9±0.9 | −3.3 | 2.2 |
| Femur neck T-score | 126 | −1.6±1.2 | −4.3 | 2.4 |
| BMI | 126 | 24.9±3.7 | 17.6 | 35.4 |
| CD4+ cell count nadir | 124 | 313.8±226.2 | 5.0 | 1260.0 |
| CD4+ cell count | 120 | 440.9±215.7 | 25.0 | 1260.0 |
| HIV RNA (log10 copies/mL) | 93 | 5.2±1.0 | 1.7 | 7.7 |
DEXA showed that the BMD of 77.7% (98/128) of the patients was decreased, 30 (23.8%) patients having osteoporosis, 68 (53.9%) having osteopenia. Twenty-nine of the 106 male patients (27.4%) had osteoporosis, 53 (50%) had osteopenia. One (5%) of the 20 female patients had osteoporosis, 15 (75%) had osteopenia/osteoporosis. Grouping of patients according to sex, did not show any differences of low BMD (p=0.794). However, males showed significantly higher rates of osteoporosis than females (p=0.042).
Statistically, there was no correlation between osteopenia/osteoporosis and age (p=0.166). According to BMI criteria, 75 (59.5%) patients had normal weight, 36 (28.6%) were overweight, 14 (11.1%) were obese, only 1 (0.8%) was underweight. One (1%) of the 98 patients with the diagnosis of osteopenia/osteoporosis was underweight, 60 (61.2%) had normal weight, 26 (26.5%) were overweight, 11 (11.3%) were obese. No significant relationship was found between BMI and osteopenia/osteoporosis (p=0.780).
In 96 patients, 25(OH)D levels were evaluated during the period of June 2010–October 2010, when exposure to direct sunlight is at its highest levels. Of these patients, 79 (82%) were males, median age was 40.1 years (range, 20–70). Fourteen (14.6%) patients had deficient, 66 (68.8%) had insufficient and 16 (16.7%) had sufficient levels. While the mean age of deficient/insufficient patients is 40.04±1.21, the mean age of sufficient patients is 43.56±3.19 and no correlation was observed between age and vitamin D levels (p=0.254). Seven out of 17 female patients whose 25(OH)D levels were evaluated had veiled dressing style. We found the level of 25(OH)D significantly low in women with veiled dressing style (17.0±7.9nmol in veiled and 33.9±22.0nmol in unveiled patients, p<0.001). Twenty-two percent of BMD normal cases and 15% of osteopenia/osteoporosis cases had normal 25(OH)D levels. No relation between osteopenia/osteoporosis development and 25(OH)D levels was shown statistically by Mantel Haenzel test (p=0.283). Among 19 patients who were not on HAART, 25(OH)D levels were deficient in three (15.8%) and insufficient in 14 (73.7%). Among 77 patients who used HAART, 25(OH)D levels were found to be deficient in 11 (14.3%), insufficient in 52 (67.5%). Vitamin D levels were similar in the two groups. Smoking, heavy alcohol use and injection drug use were not significantly associated with osteopenia/osteoporosis (p=0.09, p=0.816, p=0.724 respectively).
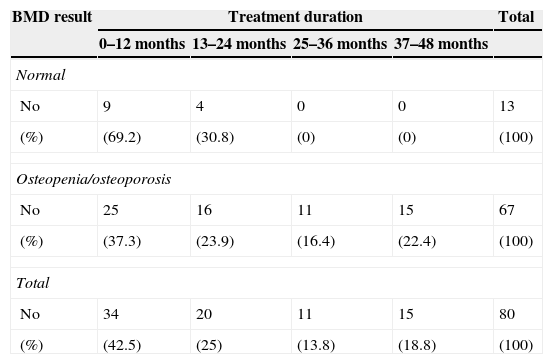
Statistical association between HIV RNA levels at first patient admission and osteopenia/osteoporosis was observed (p=0.034). Osteopenia/osteoporosis was present in 37 of 45 of patients with nadir CD4+ count of <200; 25 of 37 patients with nadir CD4+ level of 200–350, and 34 of 42 patients with nadir CD4+ level of >350. There was no statistical difference among these three groups (p=0.229). Also, among 120 cases in whom CD4+ count was assessed before BMD measurement, osteopenia/osteoporosis was present in 9 of 15 patients with CD4+ count of <200, 19 of 24 patients with 200–350 and 66 of 81 patients with CD4+ count of >350. No statistical significance was found among these three groups (p=0.097). Eighty of 126 patients (63.5%) were treated with HAART. Osteopenia/osteoporosis was found in 67 (83.7%) of the 80 cases using HAART, and 31 (67.3%) of 46 cases not on HAART (p=0.033). Osteopenia/osteoporosis rate was significantly associated with time elapsed on HAART (p=0.008) (Table 2).
Association between osteopenia/osteoporosis and duration of HAART.
| BMD result | Treatment duration | Total | |||
|---|---|---|---|---|---|
| 0–12 months | 13–24 months | 25–36 months | 37–48 months | ||
| Normal | |||||
| No | 9 | 4 | 0 | 0 | 13 |
| (%) | (69.2) | (30.8) | (0) | (0) | (100) |
| Osteopenia/osteoporosis | |||||
| No | 25 | 16 | 11 | 15 | 67 |
| (%) | (37.3) | (23.9) | (16.4) | (22.4) | (100) |
| Total | |||||
| No | 34 | 20 | 11 | 15 | 80 |
| (%) | (42.5) | (25) | (13.8) | (18.8) | (100) |
Osteopenia/osteoporosis development was not associated with the nucleoside reverse transcriptase inhibitors (NRTI) backbone, lamivudine+zidovudine or tenofonir+emtricitabine (p=0.106). Likewise, neither non-nucleoside reverse transcriptase inhibitors (NNRTI) or protease inhibitors (PI) containing regimens was associated with osteopenia/osteoporosis (p=0.365).
Osteopenia/osteoporosis may be diagnosed before a fracture occurs by measuring BMD by DEXA. We evaluated BMD of HIV-infected patients using DEXA. Osteopenia and osteoporosis were diagnosed in 53.9% and 23.8%, respectively. Osteopenia rates varies from 24% to 59.5% and osteoporosis rates from 2% to 23% have been reported in HIV-infected patients.4,5 Many studies have found higher rates of osteopenia/osteoporosis among male HIV-infected patients,6,7 in contrast to our study. Although females and males had a similar age range, males showed significantly higher rates of osteoporosis than females.
Aging is a well-established risk factor for reduced BMD in the general population, as well as in HIV-infected patients.4,7 In our series, osteopenia/osteoporosis incidence was non-significantly associated with age. Seventy-seven percent of our patients, of whom 55.5% were in the range of 20–40 years of age, presented a decrease in BMD.
In many studies, low BMI has emerged as an independent risk factor for low BMD in HIV infected patients.4,7,8 Weight loss, wasting and malabsorbtion are associated with HIV/AIDS. Out of 126 patients 45 (35.7%) had AIDS, only one of them had low BMI, none of them had wasting syndrome. In addition, the HIV-infected population has high rates of smoking, alcohol abuse and illicit drug use, which are risk factors for low BMD. But these parameters have not accounted for the higher osteopenia/osteoporosis rate in our patients.
In addition to immune regulation, vitamin D has the most important role in calcium homeostosis and bone health. Serum 25(OH)D levels reflect endogenously synthesized vitamin D and that obtained from the diet. Normal reference values depend on factors such as sunlight exposure, season, latitude, skin pigmentation, age, and dietary vitamin D intake. Istanbul is located at 41°N 28°E where sufficient sunlight is available to provide adequate ultraviolet B exposure between June and October. Thus, in order to assure statistical reliability of the study 25(OH)D levels of these 96 patients were studied during the sunny season in Istanbul, Turkey. As a consequence of low sun exposure, in this study 25(OH)D level was found significantly low in women with veiled dressing style. In HIV-infected patients, low 25(OH)D levels are likely a combination of both traditional risk factors and HIV-specific and HAART-specific contributors.9 Cozzolino et al. 10 demonstrated the inhibition by PIs of the 25-hydroxylase and the 1α-hydroxylase involved in vitamin D metabolism. Ellfolk et al. 11 showed the inhibition of the 25-hydroxylase by efavirenz. Vitamin D deficiency and bone disease in HAART patients have been associated with tenofovir and PIs.12 Vitamin D deficiency and insufficiency rates were similar across different patient groups using and not using HAART. Rodriguez et al. 13 have found 25(OH)D deficiency in 10.5%, insufficiency in 36.8% among 57 unselected HIV-infected outpatients. 25(OH)D deficiency rate of our series was similar to this study (14.6%), whereas insufficiency rate was twice more often (68.8%).
The association between chronic inflammatory conditions and low BMD is well documented and receptor activator of NFkB ligand (RANKL), the key mediator of osteoclast activity, is produced by activated T cells. In HIV infection, the virus can infect both activated T cells and macrophages. HIV-infected activated T cells and osteoblasts produce RANKL, which further stimulates osteoclast activity. Specifically, HIV proteins increase osteoclastic activity and decrease bone formation by promoting osteoblast apoptosis.14 Thus, many studies showed association between low BMD and higher levels of HIV viremia in HAART naive patients.15,16 In our study, viral load level was statistically higher in patients with osteopenia/osteoporosis in line with other studies. However, Cazanave et al. 7 showed that low HIV viral load and low CD4+ lymphocyte nadir were independent risk factors for low BMD, suggesting an effect of therapy rather than the disease itself. On the other hand, Collins17 had not found an association between abnormal BMD and CD4+ cell count nadir. In our study, there was no correlation between either CD4+ cell count nadir or CD4+ cell count before BMD measurement and osteopenia/osteoporosis.
Current knowledge suggests that both HIV and HAART are likely to contribute to osteopenia/osteoporosis in HIV-infected patients. During antiretroviral therapy, especially at the beginning, there is an accelerated bone mineral loss associated with bone resorbtion markers. Immune reconstitution may play a role in early antiretroviral therapy-related bone loss. Initiation of HAART induces a marked loss of BMD (2–6%) within the first two years, regardless of the initial choice of antiretroviral therapy.18 In this study, it had been observed that, the use of HAART and the duration of therapy were significantly related to osteopenia/osteoporosis. This finding could be due to HAART use, but it also could be advanced HIV infection necessitating HAART use. However, BMD reduction with time on therapy suggests the effect of HAART on bone density. Many authors indicate PIs as the responsible drug for low BMD, whereas others could not find any difference among the various drugs used in HAART.4,12,19 Some PIs have been shown to inhibit osteogenesis and increase osteoclastogenesis. Among the NRTIs, of greatest interest for most clinicians, tenofovir has been the most frequently associated with bone loss. Tenofovir may affect bone indirectly through renal proximal tubule toxicity, resulting in phosphate wasting and increased bone turnover. Efavirenz and PIs may affect BMD directly through Vitamin D metabolism.20 Patients in this research, used either zidovudine/lamivudine or tenofovir/emtricitabine of the NRTI group, lopinavir/r of the PI group, efavirenz of the NNRTI group. There was no difference between drug groups in the sense of osteopenia/osteoporosis development in this study.
The two possible limitations of this study would be the small sample size and low heterogeneity of the patients. These were unavoidable due to the small number of total HIV/AIDS patients in Turkey.
In conclusion, this study shows a very high prevalence of bone mass reduction in Turkish HIV-infected patients, especially in young men. High viral load, use of HAART and duration of therapy contributed to the increased prevalence of osteopenia/osteoporosis in our HIV infected population. It is suggested that a monitoring of bone health should become part of routine care for all HIV-positive patients. With effective treatments for osteopenia/osteoporosis available, it should be possible to identify high-risk individuals and prevent fragility fractures in HIV-infected patients.
Conflicts of interestThe authors declare no conflicts of interest.