Data regarding Hepatitis B and C viruses (HBV and HCV) prevalence among military personnel in Brazil are lacking, but the work-related risk of exposure can be high. The objective of this study was to estimate the seroprevalence of HBV and HCV and the risk factors associated to HBV exposure among Brazilian military personnel.
MethodsA cross-sectional study was conducted and included 433 male military adults aged 18–25 years old working in Rio de Janeiro during October 2013. All individuals completed a questionnaire to assess their risk of exposure and provided a blood sample to HBV and HCV testing.
ResultsNone of the participants presented HBsAg or anti-HBc IgM, 18 (4.1%) were positive for total anti-HBc, 247 (57.0%) were positive for anti-HBs, and 3 (0.7%) were anti-HCV reactive. The majority of military personnel with past HBV infection (anti-HBc reactive) and HBV immunity (anti-HBs reactive) had a history of prior dental procedures (88.9% and 77.3%), consumption of alcohol at least once a week (50% and 55.9%), and practiced oral sex (61.1% and 58.3%, respectively). In addition, anti-HBc positivity was common among individuals with a history of surgery (44.4%) and practice of anal sex (50%). At univariate analysis, age group was associated to anti-HBc and anti-HBs positivity.
ConclusionsLow rates of HBV and HCV infection were observed among Brazilian military personnel in comparison to the general Brazilian population. HBV immunity rates were relatively low indicating the need for vaccination campaigns in this group.
Hepatitis B and C viruses (HBV and HCV) share parenteral route as a common mode of transmission. Worldwide, approximately 240 million people are chronically infected with HBV and 130–150 million with HCV.1,2 A population-based multicentric, epidemiological survey was conducted in the general population across the five geographic regions of Brazil and found an overall HBsAg seroprevalence rate of 0.37%, 7.4% of anti-HBc, and 1.38% of anti-HCV among individuals aged 10–69 years.3,4
Prevalence studies of these blood-borne diseases showed certain risk groups and behaviors that should be considered as reasons for concern and taken into account when designing a more appropriate epidemiological investigation. In this context, anti-HBc reactivity has been reported to be 1.7% among health professionals, 5.9% among beauticians, and 12.8% among recyclable waste collectors.5–7 Anti-HCV seroprevalence has a narrower range: 0.2% among children, 1.3% among crack users, and 1.4% among truck drivers.8–10
Young men are group in whom it is very important to recognize risk behaviors associated with parenterally transmitted diseases. Military personnel offer a reachable and often nationally representative sample for disease surveillance. In Brazil, military service is compulsory for one year for men aged 17–20 years from all social classes. Brazilian military personnel are being sent to countries that present high estimated prevalence rates for HBV and HCV infection, such as Colombia where HBV prevalence was 18.6%11 and Haiti with HCV prevalence of 4.4%.12 This group of individuals could be more exposed to transmissible infectious diseases due to their missions.13,14
HBV immunization was included in the Brazilian vaccination schedule for newborns in 1996 and was also recommended to military personnel in 2010.15 In Brazil, the coverage of hepatitis B vaccination among children less than 18 months old varies from 80 to 95% according to socioeconomic status.16 Among young adult males in the Air Force in South Brazil, 84% of them reported a three-dose schedule of HBV vaccination while 66.9% of fire-fighters from Central Brazil had serum markers of HBV immunity.17,18 Thus, in face of the paucity of data regarding HBV and HCV markers of infection among military personnel in Brazil, this study was conducted to estimate the prevalence rates of HBV and HCV markers and risk factors in military personnel serving at a military unit in Rio de Janeiro City, in southeast Brazil.
MethodsStudy populationThis is a cross sectional, seroprevalence study and consisted of 433 military male personnel in the age range of 18–25 years old. All of them belonged to the largest military unit considered to be a central point for recruits and officers education in Rio de Janeiro State and one of the oldest units of Brazil. In this unit, approximately 1200 individuals are serving in the Military, the majority of them being conscripts.
In Brazil, military service is compulsory and young men have to draft for serving in the armed forces, most of them in the Army, when they turn 18, independent of level of education or socio-economic status. After concluding the recruitment process, those conscripts who were considered suitable by a selection commission begin basic military training at different military units. Those willing to pursue a military career will remain in service.
The recruitment for this study was began in October 2013 and all individuals serving at the aforementioned unit were invited to participate in the study soon after their arrival to that military unit. Study participants were male, aging 18 years or more, and registered in the military service. Those not consenting to participate were excluded.
QuestionnaireA standard questionnaire was submitted to the study subjects by the team of this study before blood collection. The questionnaire inquired about socio-demographic characteristics of the individuals (age, gender, educational status, income level, history of previous hepatitis) and risk factors for hepatitis B and C [history of blood transfusion or blood products, surgery, intravenous drug use, haemodialysis, dental procedures, acupuncture, tattooing, piercing, alcohol consumption at least once per week, sexually transmitted diseases (STDs), sexual orientation, number of sexual partners, condom usage, practice of oral and/or anal intercourse, exposure to manicure/pedicure who used non-sterilized instruments, and sharing personal care items such as toothbrushes, razors/blades, nail clippers or scissors].
Information on HBV vaccination (vaccination status and number of doses received) was collected through a self-report method since vaccination cards or medical charts were not available for consultation at the time of enrolment into the study.
Blood sampling for detection serological markers of viral hepatitisA blood sample (5mL) was taken from each subject by venipuncture using a vacutainer device. The sample was allowed to clot for serum recovery and stored at −20°C until analysis.
Serum samples were tested for HBsAg, anti-HBc IgM, total anti-HBc, anti-HBs, and anti-HCV using commercial enzyme-immunoassay (ELISA) kits (Diasorin, Italy), according to the manufacturer's instructions. Samples found to be negative on the preliminary screening were considered seronegative and samples initially tested borderline or positive were retested using the same assay in order to confirm these results.
Data collection and analysisThe prevalence rates of HBV and HCV markers were calculated for the total study population. Continuous variables were reported as the mean±standard deviation. Descriptive statistics were generated for the responses, and the chi-squared test (χ2) for independence or for trend was used to assess the association of categorical variables and anti-HBc and anti-HBs status by using the Statistical Package for the Social Sciences (SPSS for Windows, release 20.0; SPSS, Inc., Chicago, IL, USA). The results were considered statistically significant when p<0.05.
Ethical considerationEthical approval was given by the Fiocruz Ethical Committee, Rio de Janeiro, Brazil. All participants were given verbal explanation on the objectives and methodology of the research and were ensured about confidentiality, that their participation was voluntary, and they had full right to withdraw from the study at any time. Subjects were included in the study after obtaining signed informed consent.
ResultsDemographic and risk factors characteristicsThe socio-demographic characteristics of 433 military personnel are shown in Table 1. All individuals were male and most of them aged 18–25 years (75%), had secondary education (64.9%), and reported a monthly family income up to U$ 850 dollars (48.5%).
Demographic characteristics of Brazilian military personnel according to anti-HBV antibodies (total anti-HBc and anti-HBs) seropositivity (n=433).
| Characteristics | Totaln=433 (%) | Total Anti-HBc positiven=18 (%) | p-value | Anti-HBs positiven=247 (%) | p-value |
|---|---|---|---|---|---|
| Age group (years) | 0.001 | 0.004 | |||
| 18–25 | 325 (75.0) | 10 (55.5) | 194 (78.5) | ||
| 26–32 | 42 (9.7) | 0 (0.0) | 27 (10.9) | ||
| 33–40 | 32 (7.4) | 3 (16.7) | 12 (4.9) | ||
| >40 | 34 (7.9) | 5 (27.8) | 14 (5.7) | ||
| Education level | 0.853 | 0.864 | |||
| Pre-school | 10 (2.3) | 2 (11.1) | 8 (3.2) | ||
| Primary school | 105 (24.3) | 1 (5.5) | 59 (23.9) | ||
| Secondary school | 281 (64.9) | 14 (77.8) | 156 (63.2) | ||
| College | 33 (7.6) | 0 (0.0) | 22 (8.9) | ||
| Post-college | 4 (0.9) | 1 (5.5) | 2 (0.8) | ||
| Family income | 0.529 | 0.540 | |||
| 210 (48.5) | 9 (50.0) | 121 (49.0) | |||
| U$ 851.00–1695.00 | 147 (33.9) | 4 (22.2) | 79 (32.0) | ||
| U$ 1696.00–2542.00 | 41 (9.5) | 3 (16.7) | 24 (9.7) | ||
| U$ 2543.00–3390.00 | 23 (5.3) | 1 (5.5) | 16 (6.5) | ||
| >U$ 3391.00 | 12 (2.8) | 1 (5.5) | 7 (2.8) | ||
Only seven individuals reported a history of previous hepatitis, and only one had reported HBV infection. Regarding HBV vaccination, 139 informed to be vaccinated but only 32 reported three-dose schedule.
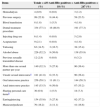
The risk factors of study participants are shown in Table 2. The most prevalent risk factors reported by the participants were prior dental treatment (77.1%), practice of oral sex (59.1%), and alcohol consumption (52.2%).
Factors related to anti-HBV antibodies (total anti-HBc and anti-HBs) positivity among Brazilian military personnel (n=433).
| Items | Totaln=433 (%) | Anti-HBc positiven=18 (%) | Anti-HBs positiven=247 (%) |
|---|---|---|---|
| Hemodialysis | 4 (0.9) | 0 (0.0) | 3 (1.2) |
| Previous surgery | 99 (22.9) | 8 (44.4) | 58 (23.5) |
| Blood transfusion | 8 (1.8) | 1 (5.5) | 4 (1.6) |
| Dental treatment procedure | 334 (77.1) | 16 (88.9) | 191 (77.3) |
| Injecting drug use | 6 (1.4) | 0 (0.0) | 5 (2.0) |
| Acupuncture | 9 (2.1) | 0 (0.0) | 4 (1.6) |
| Tattooing | 63 (14.5) | 3 (16.7) | 38 (15.4) |
| Alcohol abuse | 226 (52.2) | 9 (50.0) | 138 (55.9) |
| Previous sexually transmitted disease | 12 (2.8) | 0 (0.0) | 8 (3.2) |
| More than one sexual partner per year | 140 (32.3) | 5 (27.8) | 90 (36.4) |
| Unsafe sexual intercoursea | 181 (41.8) | 6 (33.3) | 90 (36.4) |
| Oral intercourse practice | 256 (59.1) | 11 (61.1) | 144 (58.3) |
| Anal intercourse practice | 145 (33.5) | 9 (50.0) | 87 (35.2) |
| Sharing personal care itemsb | 30 (6.9) | 1 (5.5) | 18 (7.3) |
| Earring/piercing | 154 (35.6) | 5 (27.8) | 92 (37.2) |
| Manicure/pedicure | 70 (16.2) | 2 (11.1) | 41 (16.6) |
Only six individuals reported to be non-injectable drug users at the moment of the study. However, 22 individuals reported previous intravenous drug use, and 10 of them used only once. Regarding sexual orientation, 427 (98.6%) individuals said to be heterosexual, and 256 (59.1%) had a regular partner. One hundred eighty-one (41.8%) individuals never used condom during sexual intercourse, 12 (2.8%) had a history of sexually transmitted infections (STD), 256 (59.1%) and 145 (33.5%) reported oral and anal intercourse, respectively.
Thirty individuals admitted to share personal instruments, like toothbrushes, razor or blades. Sixty-three conscripts had tattoo, 70 usually use manicurists, and 154 had piercing.
Viral hepatitis prevalence and risk factorsAll individuals tested negative for HBsAg or anti-HBc IgM markers; 18 (4.1%) were anti-HBc/anti-HBs reactive, indicating previous HBV infection, and 247 (57.0%) individuals presented isolated positivity for anti-HBs, showing HBV immunity secondary to vaccination. Anti-HCV was detected in three individuals, resulting in an overall prevalence of 0.7% and two of them reported illicit substances use in the past.
The majority of individuals presenting past HBV infection and HBV immunity had history of dental procedures (88.9% and 77.3%, respectively), consumption of alcohol (50% and 55.9%, respectively), and practice of oral sex (61.1% and 58.3%, respectively). In addition, anti-HBc positivity was common among individuals presenting history of surgery (44.4%) and practice of anal sex (50%). At univariate analysis, age-group were associated to anti-HBc and anti-HBs positivity (Table 1).
DiscussionStudies conducted to determine HBV and HCV prevalence have been widely investigated in many occupational groups6,8,18, but few data are available on its prevalence among military personnel. The present study demonstrated that 57% of Brazilian military individuals had serological evidence of HBV immunity, probably due to the inclusion of HBV vaccination in childhood immunization program, since most of individuals were young. The same rate of HBV immunity was observed among air force personnel from South Brazil,17 but this rate is lower than the rate reported among Saudi Arabia soldiers (57.5%), fire-fighters from Central Brazil (66.9%), Spanish military personnel (78.3%).13,18,19
Nowadays the coverage of hepatitis B vaccination among children less than 18 months of age varies from 80 to 95%,16 but the low prevalence of HBV immunity observed in the present study is probably due to the fact they were the first generation of compulsory vaccination.
A person is considered immune to HBV when anti-HBs levels are equal or greater than 10mIU/mL in serum, which may be acquired through HBV infection or post vaccination. Ninety-eight percent of infants achieve seroprotection after HBV vaccination with a three-dose schedule.20 However, after completion of the vaccine schedule, anti-HBs titers decline and may fall below this threshold, sometimes to undetectable levels.21 In the present study, 32.1% of individuals reported previous HBV vaccination but only 7.4% of them had completed the schedule, and 21.0% of them presented anti-HBs reactivity (anti-HBs levels equal or greater than 10mIU/mL in serum). HBV immunity rates may be 21.0% among those who reported a 3-dose schedule, 32.1% among those who reported HBV vaccination and 57% considering those with detectable anti-HBs. These results show the importance of anti-HBs testing in order to confirm HBV immunity since self-reported vaccination could not be confirmed.
Military personnel are more exposed to transmissible infectious diseases due to their missions.13,14 These individuals are far from home and exposed to several infectious agents what could have an impact over virus heterogeneity since they can import new variants. These professionals may serve as a source of infection, especially for STD since military installations usually attract gatherings of sex workers.
In the present study, no conscript were positive for HBsAg or anti-HBc IgM markers while HBsAg prevalence was found to vary from 0.3% among Greek military recruits, 2.8% among Turkish recruits, and 4% among Saudi Arabia soldiers.22–24 The finding that all individuals in the present study tested negative for HBsAg could be explained, at least in part, by the age less than 25 years of most study subjects. HBV vaccination was became part of the vaccination schedule to all newborns in Brazil in 1996 and was extended to individuals aged up to 20 years in 2001.
HBV past infection was observed in 18 (4.1%) young military males, a frequency lower than that observed among Saudi Arabian soldiers (13.2%)19 but higher than that reported among military personnel from Greece (1.68%), and Spain (0%).13,22 Anti-HBc reactivity was associated with age-group. The same was observed among recyclable waste collectors,7 indicating that over time, there is a greater trend of acquiring HBV infection related to sexual and parenteral exposures.
Anti-HCV prevalence was 0.7% among Brazilian military personnel, a prevalence similar to that reported by studies conducted among Afghan National Army recruits (0.8%)14 and Peruvian Air Force, (0.2%),24 but this rate was lower than that documented among Pakistan Military Force (3.13%).25 Anti-HCV prevalence among Brazilian military personnel was lower than the rate (1.38%) reported for the general Brazilian population4 and suggests that conscripts are not under higher risk for HCV infection. In addition, most anti-HCV positive individuals reported previous intravenous drug use, suggesting that this risk factor could be the mode of transmission in this small group of conscripts. Our results reinforce the need for education programs in order to avoid risk practices for HCV acquisition in this population.
The present study presents some limitations. First, it may be affected by selection bias, as the participants were predominantly healthy young adult males. Therefore, the results cannot be extended to the Brazilian general population or to special populations at high risk for HBV and HCV infection, such as intravenous drug users, sex workers, and hemodialysis patients. Second, validity of self-reporting data such as HBV vaccination and history of viral hepatitis might be compromised by recall bias since these informations were not obtained by consulting the conscripts’ records. Since anti-HBs levels could decline over time in vaccinated individuals, it is possible that some conscripts with undetectable levels of anti-HBs have already been, in fact, vaccinated.
Finally, vaccination programs are important in this group, since these individuals are more exposed to viral infections during their duties. In this context, they can play an important role on virus epidemiology either by importing or exporting HBV variants.
ConclusionIn conclusion, this study shows a low prevalence of HBV and HCV infection among Brazilian military personnel, reflecting the success of universal immunization toward the eradication of HBV transmission. The rate of HBV immunity was relatively low in this group indicating the need for vaccination campaigns targeting these professionals and the importance of prevalence studies for HBV and HCV infection in order to design effective prevention and control programs.
Conflicts of interestThe authors disclose no current or potential conflict of interest, including any financial, personal or other relationships with people or organizations, within two years of the beginning of this study that could inappropriately influence the study.
Source of supportThis research was supported by the Support Foundation for Research of Rio de Janeiro State (FAPERJ), Brazilian National Counsel of Technological and Scientific Development (CNPq) and the Oswaldo Cruz Foundation (FIOCRUZ).
The authors wish to thank Islene Azevedo de Souza e Silva, Maristella Matos da Costa, Nayhanne Tizzo de Paula, Sergio de Araujo Pereira, Vanessa Duarte da Costa, Vithoria Vidotti Neves for technical assistance in data collection.