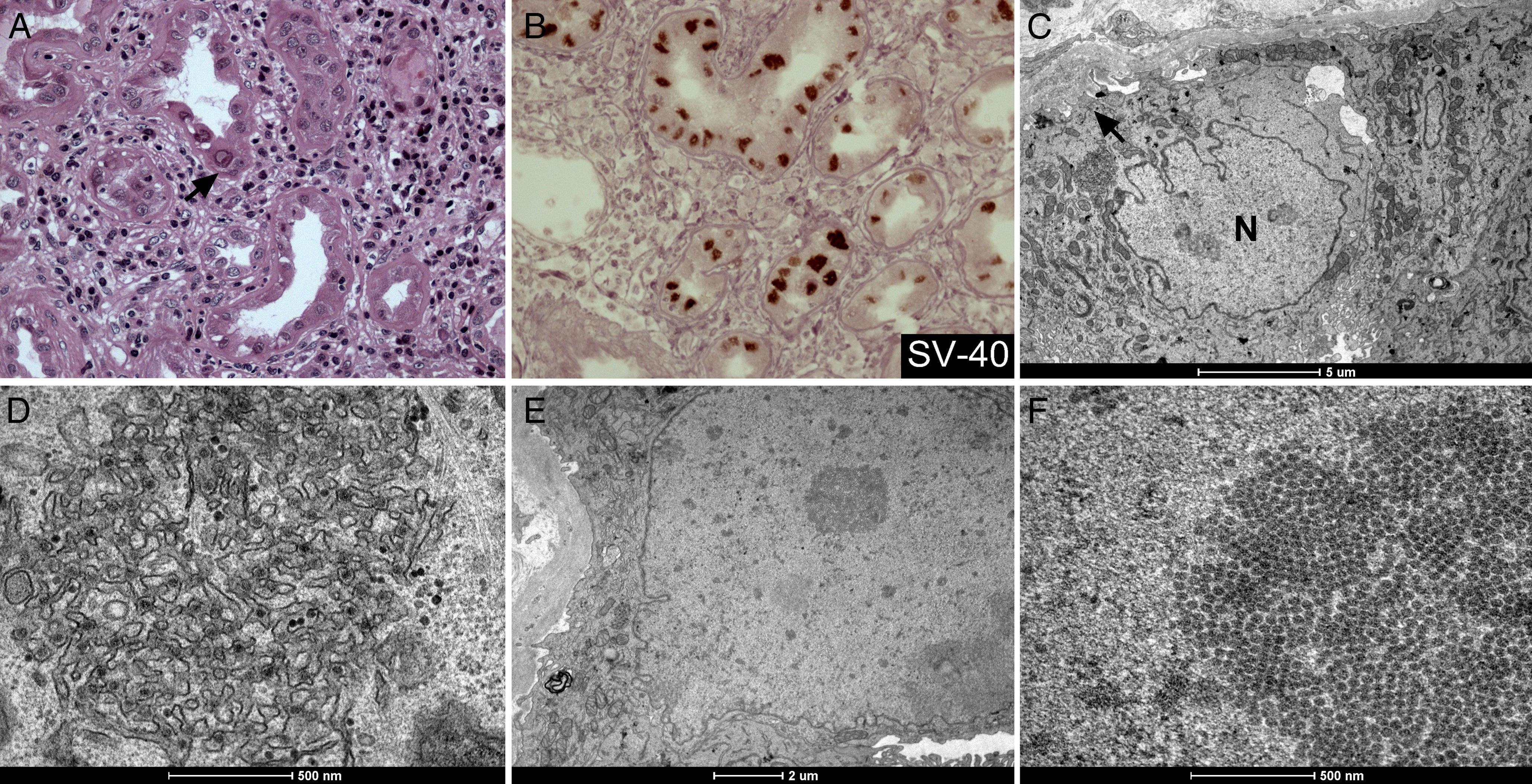
A 32-year-old-man, an Alport Syndrome carrier X link genetic disorder,1 has developed renal insufficiency and has been on peritoneal dialysis for 10 months. In June/2011, he underwent a kidney (cadaver) transplantation with 3 panel mismatch, reactivity of lymphocytes Class I and II 0% and MIC-A negative. The surgery was successful and the patient presented normal nitrogen metabolites (1.2mg/dL creatinine and 39mg/dL urea), but four months later the patient was again hospitalized due to cytomegalovirus systemic infection accompanied with graft dysfunction (creatinine of 4.3mg/dL). Despite the 14 days of treatment with ganciclovir, the lowest creatinine level was 3.3mg/dL. One year thereafter the patient presented gingival infection and worsened renal function, being treated as an upper respiratory viral infection with good response. He had optimized immunosuppressive drugs with therapeutic target levels and three months later renal function had worsened and he had an estimated glomerular filtration rate of 16mL/min/1.73m2. Renal biopsy was performed in order to better understand the graft dysfunctions which revealed accentuated tubular interstitial inflammation with some epithelial tubular nuclear atypia characterized by nuclear inclusion, cariomegaly and frosted glass appearance (Fig. 1A). Polyomavirus (BK virus) infection was then suspected and SV40 immunohistochemistry and C4d (an important marker of humoral rejection for differential diagnosis) were performed. SV40 showed diffuse nuclear positivity (Fig. 1B) in the tubular epithelial cells and C4d was negative in the peritubular capillaries walls, discarding humoral rejection. To confirm polyomavirus infection, biopsy specimens were submitted to transmission electron microscopy (TEM). Many viruses were accumulated in tubule-vesicular aggregates close to nucleus (Fig. 1C and D). These aggregates were in continuity with rough endoplasmastic reticulum. Some nucleus showed accumulation of viral particles with a diameter of 45nm in loose groups or in dense crystalline arrays, characteristic of the mature infection (Fig. 1E and F).2 Despite some tubular cells not showing any viral particle, we observed cytoplasmic changes indicating cellular infection such as loss of brush border, nucleus irregularly shaped and loss of nuclear polarity. Nowadays, there is no effective treatment for the BK virus infection. The infection is still poorly understood and studies on ultrastructural levels have important implication in treatment and prevention of BK virus-associated nephropathy.
Polyomavirus infection in graft transplant morphology. (A) Epithelial tubular nuclear inclusion (arrow) by H&E; (B) SV40 nuclear staining (brown) by immunohistochemistry; (C and D) viruses aggregates in rough endoplasmastic reticulum (arrowhead) close to nucleus (N) by TEM; (E and F) Viral particles organized as crystalline arrays in nucleus by TEM.
The authors declare no conflicts of interest.