Plesiomonas shigelloides isolated from water in Brazil was previously described as a hemorrhagic heat-labile cytotoxic-enterotoxin producer. We purified this toxin from culture supernatants using ion metallic affinity chromatography (IMAC) followed by molecular exclusion chromatography. The pure toxin presented molecular mass of 50kDa and isoelectric point (pI) around 6.9 by 2D electrophoresis. When injected intravenously, the purified cytotoxic-enterotoxin induced also severe spasms followed by sudden death of mice. Hence, we entitled it as lethal cytotoxic-enterotoxin (LCE). The presence of membrane vesicles (MVs) on cell surfaces of P. shigelloides was observed by scan electron microscopy (SEM). From these MVs the LCE toxin was extracted and confirmed by biological and serological assays. These data suggest that P. shigelloides also exports this cytotoxic-enterotoxin by membrane vesicles, a different mechanism of delivering extra cellular virulence factors, so far not described in this bacterium.
Plesiomonas shigelloides is an aquatic microorganism recognized as human and animal enteropathogen by epidemiological evidence.1,2 Despite the existence of several reports regarding the detection of enterotoxic and cytotoxic activities, the virulence mechanisms related to the enteric infections are not yet completely defined.3–5 Sanyal et al.6 described enterotoxicity in live cultures and culture filtrates of P. shigelloides in rabbit ileal loop test, where the culture filtrates caused dislodgement of CHO and mouse adrenal tissue-culture cells. Its enterotoxicity was not affected when heated at 121°C for 10min. Gardner et al.7 described the production of a heat-labile cholera enterotoxin-like protein and Okawa et al.8 described a thermostable cytotoxin, which is composed of three LPS-binding proteins, serologically related to the cholera enterotoxin. Falcón et al.2 described that P. shigelloides, isolated from water, produced a heat-labile vacuolating activity over several cell line cultures, provoking enterotoxic activity, in suckling mouse test. The initial objective of this study was to understand the virulence factors produced by this strain (P. shigelloides 9P3-1).
It is known that bacteria outer membrane is not only a surface component that keep them in contact with the environment but it is also responsible for controlling the flux of nutrients what must enter or leave the microorganism cell. The outer membrane can release membrane vesicles (MVs) which are used as vehicle for or a virulence factor itself. These MVs are formed by the bulging in any part of the membrane structure, resulting in spherical fragments of different sizes, which are spread in the environment. More than presenting outer membrane components, as phospholipids, proteins, and lipopolysaccharides (LPS), they contain periplasmic contents.9,10
A wide variety of Gram-negative bacteria are known to release MVs, which contain enzymes, toxins, adhesins, and DNA, mainly in some genera like Escherichia, Bacteroides, Haemophilus, Pseudomonas, and Neisseria.9,11–13 Hence, the formation of vesicles, which act as vehicles for carrying toxins, is thought to be an important virulence factor in these pathogens. STEC (Shiga toxin-producing Escherichia coli) releases MVs containing shigatoxin. Furthermore, MVs produced by E. coli O157:H7 strains may contain LPS, proteins, plasmids, linear DNA with virulence genes as eae, stx1, stx2 and uidA, as well as antibiotic resistance genes, demonstrating that vesicles act delivering genetic material and toxins to other microorganisms.14,15
In this work, we demonstrate that P. shigelloides (9P3-1) isolated from river water in Brazil exports a lethal cytotoxic-enterotoxin (LCE) by membrane vesicles. These results suggest that vesicles play an important role on the spreading of LCE to host cells during the bacterial infection.
Materials and methodsThe following study was approved by the Institutional Committee for Ethics and Care in Animal Research (State University of Campinas – UNICAMP) which certifies that the protocol No. 919-1 is in agreement with the Ethical Principles for Animal Research established by the Brazilian College for Animal Experimentation (COBEA).
Bacteria and culture conditionsAmong seven strains of P. shigelloides isolates from river water,16 the strain 9P3-1 (O4:H3) was selected for presenting the major reciprocal activity titer on Vero and CHO cells. For exotoxin production, P. shigelloides strains were grown in Luria Broth (LB, Difco Lab.) in a shaker at 150rpmmin−1 (New Brunswick Scientific Co) for 14h at 37°C.
Purification of LCE (lethal cytotoxic-enterotoxin)Culture supernatants were concentrated 6×102 times from its initial broth volume, using XM-300 and XM-50 ultra-filtration membranes (Amicon, Millipore). The concentrates were submitted to Cu2+ ion metallic affinity chromatography (IMAC) gel packed chromatography column (AKTA FPLC, GE-Amersham Healthcare), equilibrated with 50mM Tris–HCl buffer, pH 8.0, containing 1M NaCl, eluted with a 0–0.5mM linear imidazole gradient.17 The chromatographic fractions were monitored by cytotoxic activity assays on Vero cells.2 Fractions presenting cytotoxic activities were concentrated again by ultrafiltration membranes and applied to chromatography column Superdex 75 HR 10/30 (GE-Amersham Healthcare), equilibrated with 20mM Tris–HCl buffer, pH 8.0 (Sigma) in a flow of 0.05mL/min. The LCE purification was confirmed by SDS (sodium dodecyl sulfate) polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions18 with 10% polyacrylamide gels, using molecular-weight markers (Sigma) and by bidimensional (2D) SDS-PAGE analysis. Protein determination was measured using Bradford Bio-Rad Protein assays, according to manufacturer's instructions.
Cytotoxicity assaysCytotoxic activity was tested according to the methodology described elsewhere.19 Vero (African green monkey kidney), CHO (Chinese hamster ovary) and CACO-2 (human epithelial intestinal cell line) (ATCC, Rockville, MD, USA) cell lineages were grown in Eagle's MEM (Nutricell, Campinas, SP, Brazil) supplemented with 10% (v/v) of calf serum (BCS).
Cellular viability assaysThe viability assays were performed as previously described,20 using neutral red as indicator. In this assay, the lethal cytotoxic dose (CD50) was calculated as described by Welkos et al.21 Lactate dehydrogenase (LDH) assays for verification of cellular integrity were performed according to the methodology proposed by Mitchell et al.22 The kind of cell death induced by the pure cytotoxin was verified by flux cytometry analysis assay (annexin V-FITC Apoptosis Detection Kit I; BD Biosciences Pharmingen). Fluorescent Actin Staining (FAS) assay was performed as previously described using soluble Rodamine – FITC Labeled (Sigma) conjugated with Fluorescein.23
Enterotoxic activitiesSuckling mice test assaySuckling mice (neonatal BALB/c 2–3 days-old) assay was performed for enterotoxin activity accordingly to a methodology described earlier.24 After 3h, mice were killed in a CO2 chamber. Positive results were considered when ratios of intestinal weights (IW) to the remaining body weight (BW) was ≥0.08. Assays were conducted in quadruplicates.
Rabbit intestinal loop assayThe rabbit intestinal loop test was performed as described by Evans et al.25 New Zealand white male rabbit weighting 1.5–2.0kg were not fed for 24h and sedated with Phentanyl (0.005mg/kg) and Droperidol (1mg/kg) (Janssen-Cilag Farmacêutica Ltda) by intramuscular injection. Subsequently, the rabbits received an epidural analgesia with Morphine (0.1–0.3mg/kg) (Labesfal) and anesthetized by intramuscular injection with Zoletil anesthetic (20–30mg/kg) (Virbac). Morphine maintained the animal under analgesia during all the experiment. Rabbits were kept for 18h at room temperature and sacrificed thereafter. The fluid accumulation in the intestinal loop was measured as the ratio between the weight of the loop (in grams) and length (in centimeters). Ratios over 0.2g/cm were regarded as positive. Assays were performed in triplicates.
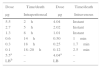
Lethal activityGroups of five adult females (5–6 week-old) BALB/c mice weighing ca. 20g (CEMIB, UNICAMP, Brazil) were inoculated intraperitoneally and intravenously (Table 1) and were observed for periods of seven days (at every 15min during the first hour of experiment and monitored periodically every 12h afterwards). Assays were conducted in triplicates.
Serological analysisSerum anti-LCE productionSpecific anti-LCE antiserum was prepared from rabbits weighing ca. revmin−1 3kg. A 100μgmL−1 dose of purified LCE (neutralized by heating 100°C during 15min) was emulsified in an equal volume of Freund's complete adjuvant (Difco) and injected intramuscularly next to the popliteal ganglia. This procedure was repeated 15 days later and two other injections with the incomplete Freund's adjuvant administered at 45th and 60th day. Two weeks after the last injection, the animals were bled.
Serum neutralization testsSerum neutralization tests were performed by adding a serial two-fold dilutions of anti-LCE to antisera anti-LT-I, anti-CT (kindly supplied by Dr. L.C. Ferreira, ICB-USP, SP. Brazil) and anti-Aerolisin.26 All antisera were diluted in PBS (1/1024 maximum dilution) and applied to 96 wells in microtiter plates. The purified LCE (2×CD50) was added to the dilutions and after 60min incubation at 37°C, 0.1mL of each mixture were transferred to the Vero cells monolayer grown in 96 wells microtiter plates. Plates were incubated at 37°C and observed for morphological alterations for up to 72h. The same tests were conducted with the contents obtained from the bacterial membrane vesicles. All the assays were performed in triplicate.
Membrane vesicles (MV)Isolation of membrane vesiclesMVs were obtained according to the methodology described by Kadurugamuwa.27P. shigelloides were cultured in 10mL of Luria-Bertani Broth (Difco Lab., USA) with shaking at 150revmin−1 (New Brunswick Scientific Co.) for 24h at 37°C. Cultures were centrifuged at 7.700×g for 15min, at 4°C. and supernatants were filtered through 0.22μm polyvinylidene difluoride membranes (Millipore Co., Bedford, MA, USA) to remove residual cells and cellular debris. Then, filtrates were ultra-centrifuged at 150,000rpm for 3h at 4°C with a 70 Ti rotor (Beckman Instruments, Inc., Fullerton, CA, USA) and MVs were then recovered from the pellet.
Polymyxin B treatment for membrane vesicles disruptionPellets of MVs obtained by ultracentrifugation were suspended with 1mL solution of phosphate buffer saline (PBS) with polymyxin B at a final concentration of 10,000IUmL−1. Suspensions were incubated at 37°C for 1h to disrupt membrane vesicles. After being centrifuged at 15,000rpm for 15min at 4°C, the obtained supernatants were filtered using 0.22μm membrane and applied to confluent monolayer Vero cells for cytotoxic activity, suckling mouse assay for enterotoxicity detection, and for mice lethal activity assay.
Electrophoresis SDS-PAGE of MVs contentsAfter ultracentrifugation, pellets containing MVs were suspended in 200μL of 10mM Tris–HCl buffer (pH 7.4) and the suspension was precipitated with 10% trichloroacetic acid (TCA). After this process, the suspension was centrifuged at 15,000rpm for 15min at 4°C in order to obtain the supernatants. One-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE was performed with the obtained supernatant according to the methodology previously described using a 10% polyacrylamide (Bio-Rad Laboratories, Hercules, CA) and 0.1% SDS gel (Merck) under reducing conditions.18
Immunoblot analysis of LCE toxin obtained from MVFor immunoblot, the electrophoresed proteins were transferred to nitrocellulose membranes Hybond-ECL (GE Healthcare Bio-sciences, São Paulo-SP, Brazil) by the method proposed by Towbin.28 Membranes were blocked in TBS-T buffer (10mM Tris–HCl, 150mM NaCl, 0.2% Tween 20, pH 7.5) with 5% of non-fat milk for 2h at room temperature and subsequently incubated with the same buffer with 1% of non-fat milk containing the antiserum anti-LCE (diluted 1:500 with TBS) for 2h at 37°C. The membrane was washed four times (10min each) with TBS-T, and finally incubated with a secondary antibody (peroxidase-conjugated anti-rabbit IgG. antibody) (Sigma) (1:1000) in TBS-T buffer with 1% non-fat milk for 90min at 37°C. After incubation, the membrane was washed four times with TBS-T. Peroxidase activity was revealed by chemiluminescent substrate (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Scan electron microscopy (SEM)Vero cells were cultured on coverslips, infected with bacterial cultures of P. shigelloides and incubated at 37°C for 30, 60 and 120min, fixed in a solution containing 50% of 2.5% glutaraldehyde (Electron Microscope Science, USA) diluted in 10mM Cacodylate buffer, pH 7.4 and 50% of cellular culture medium. After this first step, cells were fixed in a solution of 2.5% glutaraldehyde in 10mM Cacodylate buffer, pH 7.4. Slides were dehydrated by graded ethanol series, dried in a critical point dryer CPD 030 (Bal-Tec Balzers) with CO2 at 5°C under 50mbar pressure, covered with gold-palladium (25nm) (40mA – 98s) in a Sputter coater – SCD 050 (Bal-Tec – Balzer). Preparations were observed using a Scan Electron Microscope SEM (FEI Quanta 200 – with X-ray probe).
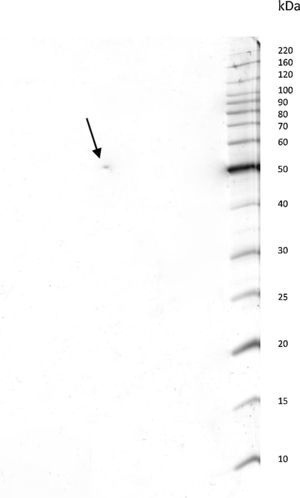
ResultsPurification of the LCEThe lethal cytotoxic-enterotoxin (LCE) produced by P. shigelloides (9P3-1) was purified from the culture supernatants by chromatographic processes through Cu2+ ion metallic affinity chromatography (IMAC) and a molecular exclusion column. Conventional SDS-PAGE and bidimensional SDS-PAGE (2D gel electrophoresis) resulted in a single protein band of 50kDa. Based on electrophoresis 2D analysis the cytotoxic-enterotoxin presents an isoelectric point (IP) around 6.9 (Fig. 1).
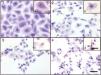
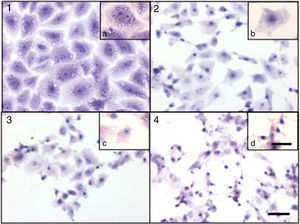
Cytotoxicity assaysCulture supernatants and the purified LCE induced cytotoxic effects on CACO-2, CHO and Vero cells (Fig. 2). Cells showed an evident and extensive vacuolation, and a change from the normal spindle shape to a round configuration, with nuclear condensation; swelling, detachment and gradual destruction of the monolayer indicating, cells death (Fig. 2).
Cytotoxic effect on Vero cells evidenced by a May Gr¿nwald-Giemsa stain. (1) Control cells; (2) 15min of cytotoxic assay; (3) 30min of cytotoxic assay; (4) 60min of cytotoxic assay. Bars in the window 4.4 represent 40μm and are valid for every big window (1–4) and 15μm for the small windows (a–d).
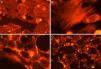
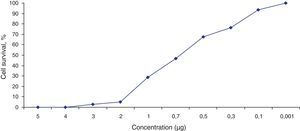
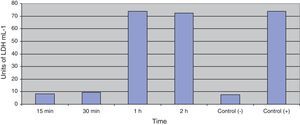
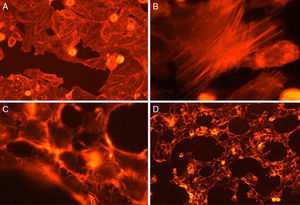
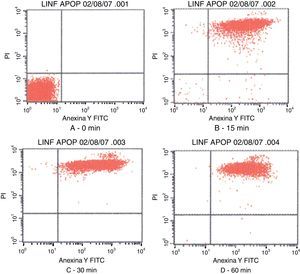
Fig. 3 shows the data obtained by the CD50 cellular test assay, where 50% of death cells as determined via NR assay, corresponding to 0.7μgmL−1 of the pure LCE in a two-hour assay according to a methodology described previously.21 Loss of cells viability by the LDH assay (Fig. 4) revealed that the purified LCE damaged the cellular membranes. FAS assay (Fig. 5) showed the formation of stress fibers, characterized by the training and thickening of actin filaments beams, responsible for the rearrangement of cytoskeleton, as well as rounding of cells, and retraction of cytoplasmic contents and extension of matrix filaments. The flux cytometry analysis assay (Fig. 6) demonstrated the cellular death resulting from apoptosis.
Contour diagram of Annexin V-FITC flux cytometry of human lymphocytes cultured by different time intervals (0, 15, 30 and 60min) with LCF (5μg). The lower left quadrants of each panels show the viable cells, which are negative for PI and Annexin V-FITC binding. The upper left quadrants contain the non-viable, necrotic cells, Annexin V-FITC negative and PI positive. The lower right quadrants represent early phase of apoptotic cells Annexin V-FITC positive and PI negative. The upper right quadrants contain the non-viable, apoptosis cells, Annexin V-FITC positive and PI negative, indicating that they were in end stage apoptosis or already dead.
Culture supernatants and the purified LCE caused a hemorrhagic fluid accumulation in the suckling mouse assay with enterotoxic ratios over 0.16; in the rabbit ileal loop test, an hemorrhagic mucus-like fluid was observed (25–30mL). This activity was lost when the LCE was heated at 60°C for 10min, demonstrating that LCE acts as a thermolabile enterotoxin.
Lethal activity of LCE in miceThe purified LCE showed a potent lethal activity in four week-old mice, when received 5.5μgmL−1 injected intraperitoneally, causing death after 2h (Table 1). Mice were prostrated, presenting bristly hair, and death. When LCE was injected intravenously, the lethal activity was very rapid, causing instantaneous death. The lethal dose determined in this test was 1.01μgmL−1 (Table 1).
Serological analysisThe cytotoxic activities of purified LCE were neutralized by homologous antiserum, presenting reciprocal titers of 1:1024, partially neutralized by anti-LT-1 produced by enterotoxigenic E. coli (ETEC) and antiserum anti-autolysin, with titers 1:256 and 1:32, respectively. However, no reaction was observed with anti-cholera toxin serum.
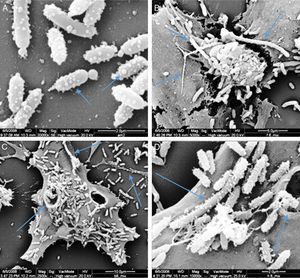
Membrane vesicles (MVs) assaysScan electron microscopy analyses showed the presence of many vesicles attached to the cell surface of P. shigelloides. Those vesicles presented spherical shape and diameters varying between 80nm and 220nm, predominantly the small ones. The presence of linked vesicles structuring a string of pearls-like were observed released by the bacterial cells (Fig. 7A). Some bacteria did not present signs of cellular division process, reaching 7.7μm, similar to the described for Proteus mirabilis.29
Scan electron micrograph shows many vesicles attached to the cells surfaces of P. shigelloides attached to Vero cells; blue arrow at A shows MVs forming several size; and the blue arrow in B shows the presence of super bacteria formation; the blue arrows at C shows bacteria invasion to Vero cells; the blue arrows at D shows super bacteria, invasion and MVs; yellow arrow at B and C shows MVs in strings.
The SDS-PAGE analyses with MVs contents revealed the presence of many proteins, among them a 50kDa protein, which was purified using the same methods applied for culture supernatants of P. shigelloides, presenting both cytotoxic and enterotoxic activities, confirming MVs as the origin of LCE.
Immunoblotting analysis of the MVs content revealed in presence of homologous rabbit antibody serum produced with the purified LCE. There was an immunological reaction against the 50kDa structure corresponding to LCE protein band.
DiscussionAcute diarrhea is mainly caused by the increased intestinal secretion, commonly as a result of infection by enterotoxin-producing organisms like enterotoxigenic E. coli or Vibrio cholerae. Alternatively, it may result from decreased intestinal absorption due to infection by microorganisms, like enteropathogenic E. coli, Shigella sp. or Salmonella sp. that damage the intestinal epithelium.30,31 Studies have described P. shigelloides as an etiological agent in diarrheal diseases, and it has been demonstrated to produce various toxins that are detected free in culture supernatants.7,2
The production of a hemorrhagic cytotoxic-enterotoxin by P. shigelloides, isolated from river water in Brazil,16 was previously studied by Falcón et al.2 They reported fluid accumulation in the suckling mouse assay, vacuolating activity on cell culture assays, induced by thermolabile culture supernatant, since it lost activity after being heated at 60°C for 15min.
The LCE from culture supernatant of P. shigelloides was purified by chromatography using IMAC and a molecular exclusion column. The 2D SDS-PAGE gel electrophoresis analysis determined that LCE presents an isoelectric point (IP) around 6.9 (Fig. 1). The purified LCE caused equal cytotoxic effects on CACO-2, CHO, and Vero cells (Fig. 2), by the NR assay presenting a CD50 of 0.7μgmL−1 of pure LCE inducing the death of 50% of cells in 2-hour assay (Fig. 3) based in the methodology described earlier.21
Loss of cell viability by LDH assay (Fig. 4) was observed, revealing that LCE causes damage to cellular membranes. All cell assays displayed an evident and extensive vacuolation, followed by a change from the normal spindle shape to a round configuration and nuclear condensation; the cells became swollen and increased in degree of cell vacuolation followed by detachment and gradual destruction of the monolayer, and death (Fig. 2). In the last decades, some authors have related the cell vacuolation effect as indicative of bacterial virulence and pathogenicity.32,33V. cholerae and Helicobacter pylori are known for their ability to produce vacuolating cytotoxins demonstrating the association between biological effects (vacuolation) and pathogenicity caused by these microorganisms.34,35
For elucidating the pathogenic potential of the LCE, FAS assay was conducted to evidence the morphological and intracellular induced alterations by the formation of stress fibers, characterized by disorders over the actin filament beams, responsible for cytoskeleton rearrangement, cells rounding and retraction of cytoplasmic contents and extension of filaments (Fig. 5).
Flux cytometry analysis (Fig. 6) revealed that LCE not only induces cytoplasmatic vacuole formation but also provokes cellular death by apoptosis. Tsugawa et al.5 also observed the effect of cellular death by apoptosis of Caco-2 cells on cytotoxic assays.
Fluid accumulation in intestinal ileal loops occurs due to the presence of enterotoxins. Sanyal et al.6 demonstrated P. shigelloides ability to provoke enteritis in test with live cultures and animal, with positive results in rabbit intestinal loop with cultures filtrates.
Culture filtrates were also positive on the suckling mouse assays, and the activity persisted after heating the supernatant at 121°C for 15min. However, Holmberg et al.36 did not observe fluid accumulation in rabbit intestinal loops using culture filtrates from 31 clinical P. shigelloides isolates.
Our purified LCE of P. shigelloides caused a hemorrhagic fluid accumulation in the suckling mouse assay and hemorrhagic mucous-like fluid in the rabbit intestinal loop test (data not shown), an indication of the strong enterotoxigenicity of this protein. This activity was lost when the LCE was heated at 60°C for 10min.
The purified LCE, when injected intraperitoneally, showed a potent lethal activity on four week-old mice, causing death after 3h. Mice were initially prostrated and progressed to death; when intravenously injected the LCE caused death in a few seconds. The lethal dose of purified LCE was determined in the mouse model and calculated as 1μgmL−1, inducing instantaneous and spasmodic death. Salvadori et al.37 described a lethal toxin from the culture supernatant of E. coli isolated from chickens with swollen head syndrome, a lethal toxin similar to that of Bacillus cereus. In our study the lethal activity was also heat-labile, since the LCE lost the lethal activity in mice after heating.
The cytotoxic activities of the LCE were neutralized by homologous antiserum but were not neutralized by the anti-cholera enterotoxin (CT) while it was partially neutralized by anti-LT-1 produced by enterotoxigenic E. coli (ETEC) (data not shown).
The LCE associated to MVs of P. shigelloides was observed after 24-hour incubation; however, the quantity of free culture supernatant toxin detectable was very low. Scan electron microscopy (SEM) analysis showed the presence of many vesicles attached to the cell surface of P. shigelloides (Fig. 7). The vesicles presented spherical shape and diameters varying between 80nm and 220nm, and the smaller vesicles were the most prevalent. Vesicles linked, as a string of pearls, were observed released from bacteria cells (Fig. 7A). We also observed structures known as super-bacteria (Fig. 7B–D) associated to bacterial growth, reaching maximum sizes of 7.7μm without any sign of cellular division.
Vesicles production was a phenomenon observed in P. shigelloides grown under prolonged time conditions in adhesion assays, and were detected by SEM. The role attributed to the vesicles is a virulence mechanism similar to periplasmic enzyme, which is exported by STEC O157 and Pseudomonas aeruginosa11,15 or used for DNA transportation12–14 or as a bacterial virulence factor like adherence and evasion of the immune system,10 and also as a component for biofilms matrices.38 Despite the data presented in some studies, the role of vesicles in transportation of different bacterial products and components has not yet been carefully analyzed. Nonetheless, these data suggest that vesicles formation by P. shigelloides represents an effective mechanism for the transport of toxin by this pathogen.
The hypothesis that the MVs are related to bacteria invasion to tissues cannot be ruled out. The bacterial invasion mechanisms into epithelial tissue are still not well understood. However, Grenier and Mayrand38 mentioned in their studies that vesicles, with their high proteolytic activity, may possibly be implicated in the subsequent epithelium penetration by the bacteria. Our observations showed that MVs are released in a great number from the bacteria cell surfaces, and their small size would enable them to cross anatomical barriers that are, otherwise, impermeable to bacterial cells. MacDonald and Beveridge39 in a study with P. aeruginosa, showed the importance of MVs attachment and predatory activities, and proposed that MVs play a key role in providing competitive advantage during bacteria growth.
SEM showed that most vesicles released from P. shigelloides are similar in size and morphology. The vesicles were observed to be close to spherical shape and ranging from 80 to 220nm in size, in contrast to the vesicles observed by Dutta et al.40 in Shigella dysenteriae type 1 strain after Mitomycin C addition to vesicles effectively released. Vesicles produced by P. shigelloides are comparable in size to those produced by other pathogens like E. coli O157:H7,14P. aeruginosa,41Neisseria gonorrhoeae42 and Bacteroides gingivalis.38
Examined with SEM some P. shigelloides did not conclude the cellular division processes, reaching cellular sizes next to the ones presented by P. mirabilis (swarming) for example, which is considered a super bacterium.28 Kadurugamuwa and Beveridge,41 suggest that P. aeruginosa is producer of VMs that contain most of the metabolites necessary for cellular natural division (with autolysins). These observations showed that the deviation of such metabolites to MVs occur probably simultaneously, underlying periplasmic components (including autolysins) that would be trapped within the blebs. When the bacteria are supposed to adhere to host cells the production of an increased number of predatory MVs may be advantageous. Therefore, it would be a peak of cellular demand during adhesion not only for autolysins to be used in cell division, but also for predatory vesicles.43
The SDS-PAGE analysis performed with the MVs content showed the presence of many proteins, including a 50kDa protein, which was abundant in the MVs and was purified from the P. shigelloides culture supernatant, presenting in both cases cytotoxic and enterotoxic activities. This toxin showed to be different from the toxin reported by Okawa et al.,8 which was described as a complex of LPS binding proteins, with molecular masses higher than 600kDa.
The immunoblotting analyses were conducted with the MVs contents and rabbit antibody serum produced with purified LCE obtained from cultured supernatants. The immunological reaction occurred against the 58kDa structure. The band obtained suggests that the biological activities induced by the cytotoxic assays were due to the same enterotoxin, which can be exported from the bacteria cell and also by MVs. A possible consequence of this characteristic is that vesicles could evade antibodies action, thus avoiding the specific antibacterial host immune defenses.44
To our knowledge, this is the first report showing that P. shigelloides released via membrane vesicles is a lethal cytotoxic enterotoxin. It is interesting to speculate which mechanisms are leading these bacteria cells to the formation of these MVs during cellular growth in vitro as well as the mechanisms for their action in vivo.
The LCE here described might be a putative intrinsic virulence factor of P. shigelloides isolated from river water in Brazil. Further studies are being conducted, in order to better understand the mechanisms of the enterotoxic, cytotoxic, and lethal activities induced by the LCE.
FundingThis work was supported by grants from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo), Brazil. The recipient was a CAPES fellowship.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Ana Stella Menegon Degrossoli (UNICAMP) for technical assistance, Oswaldo Capelo (UEL) for technical assistance to scan electron microscopy and Profa Dra Halha Ostrenski Saridakis, for correction of English.