Paracoccidioidomycosis is a systemic mycosis found mainly in South America and is the most prevalent endemic and systemic mycosis in Brazil. The purpose of this paper was to report the case of a male patient who developed peritonitis caused by Paracoccidioides spp. Forty-eight-year-old, male patient, with type I Diabetes mellitus and chronic kidney disease who was undergoing a Continuous Ambulatory Peritoneal Dialysis (CAPD) program. After eighteen months of peritoneal dialysis, the patient developed turbidity of the peritoneal fluid and was diagnosed with peritonitis. Direct mycological examination of the peritoneal fluid revealed yeasts with morphology suggestive of Paracoccidioides spp. The patient was treated with sulfamethoxazole-trimethoprim (1,600 mg/320 mg dose/day) for 61 days, but he died because a bacterial septic shock. The diagnosis of opportunistic PCM peritonitis was later confirmed by autopsy and Paracoccidioides spp. isolation. This is the first reported case of a patient on CAPD who experienced complications due peritonitis caused by opportunistic PCM.
Paracoccidioidomycosis (PCM), a systemic mycosis found mainly in South America, is the most prevalent endemic and systemic mycosis in Brazil.1,2Paracoccidioides brasiliensis is the known etiological agent of PCM; however, there are indications that other species of Paracoccidioides, including Paracoccidioides lutzii may be involved. The fungus in its mycelial form is a natural inhabitant of rural soils, but it can also survive in water and be grown in chemically composed culture media.3 Inhalation of Paracoccidioides brasiliensis propagules results in asymptomatic pulmonary infection in most individuals, with progression to disease being more likely in hosts with a poor cellular immune response.4
The clinical manifestations of the disease can be categorized into two forms. The acute/subacute form can manifest in children and young adults with signs of weight loss, fever, mild to moderate anemia, lymphadenopathy, skin lesions, and hepatosplenomegaly, corresponding to less than 10% of cases.5 The chronic form, on the other hand, accounts for more than 80% of cases and can signify a reactivation of the primary infection. PCM commonly affects men between 30 and 60 years of age who have worked in agriculture for a long time.5 The adult-type disease may present with lung involvement and ulcerated lesions on the skin and oral and nasal mucosa,5 and can spread via lymphatic or hematogenous routes to any part of the body. Infection may be opportunistic in patients with reduced cellular immunity caused by other diseases or immunosuppressant treatment.6 The best way to diagnose PCM illness is by identifying fungal elements in fresh sputum, lesion scrapes, lymph node, or biopsy of the affected organ,6 with the latter confirming the diagnosis in 97.8% of cases. Typical histology shows signs of inflammation and granulomas rich in epithelioid and giant cells containing variable amounts of fungal forms.7Paracoccidioides spp. is sensitive to most systemic antifungals. After establishing the best treatment option, patients diagnosed with PCM infection must undergo outpatient follow-up to reach the cure criteria based on clinical, mycological, radiological, and immunological parameters.6 Clinical cure is achieved when the signs and symptoms of the disease are no longer present, which includes wound healing, regression of adenomegaly, and stabilization of body weight.6 Mycological cure represents negative results on mycological tests after efficacious treatment. Immunological cure, in turn, consists of decreasing serum fungus antibodies to undetectable or in low concentrations.6
PCM can become a severe illness, and in Brazil, it is one of the leading causes of death among infectious and parasitic diseases.5
The objective of the present study was to report the case of a male patient who developed peritonitis caused by Paracoccidioides spp., and it provides a brief overview of CAPD-associated infectious peritonitis.
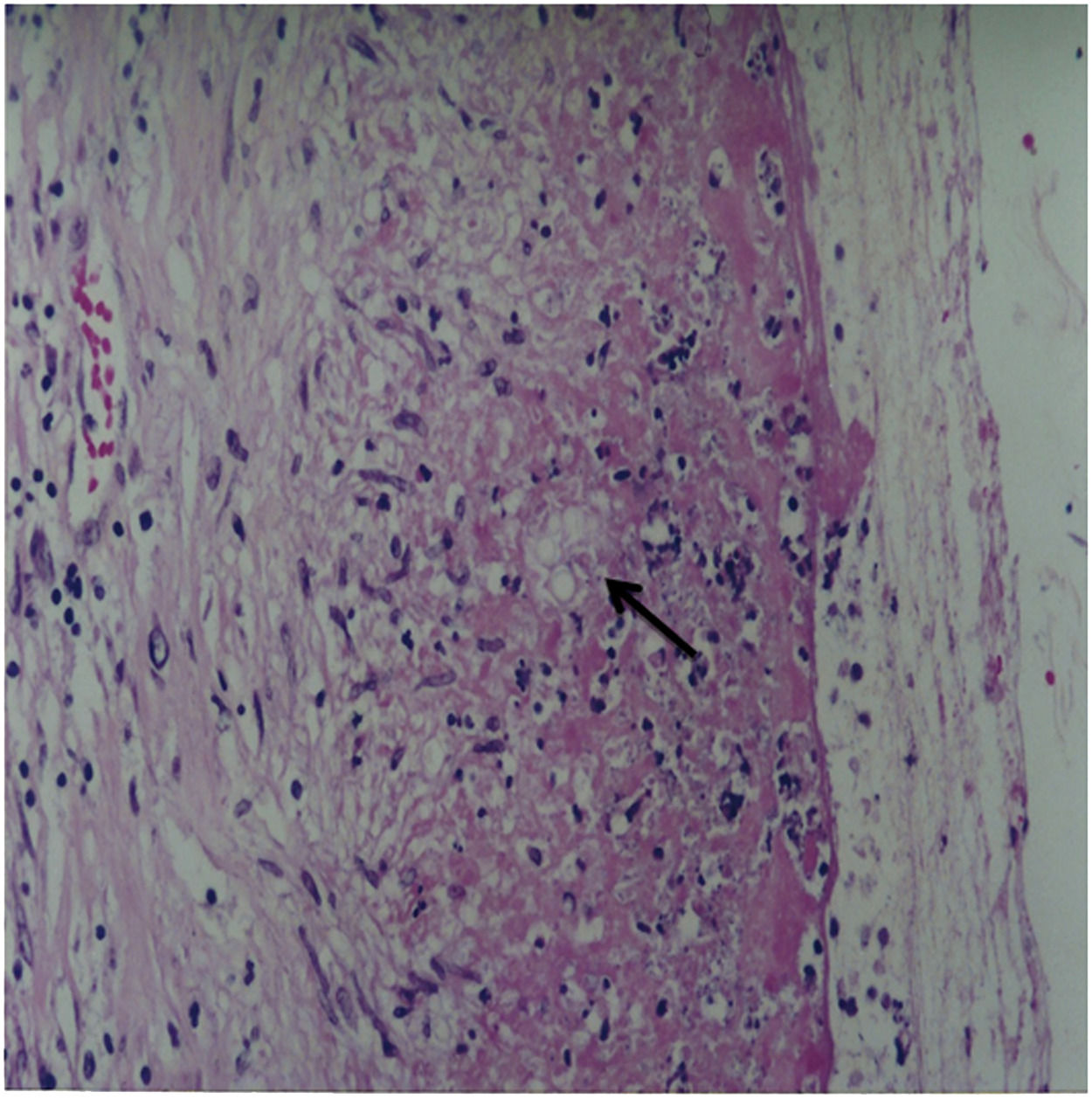
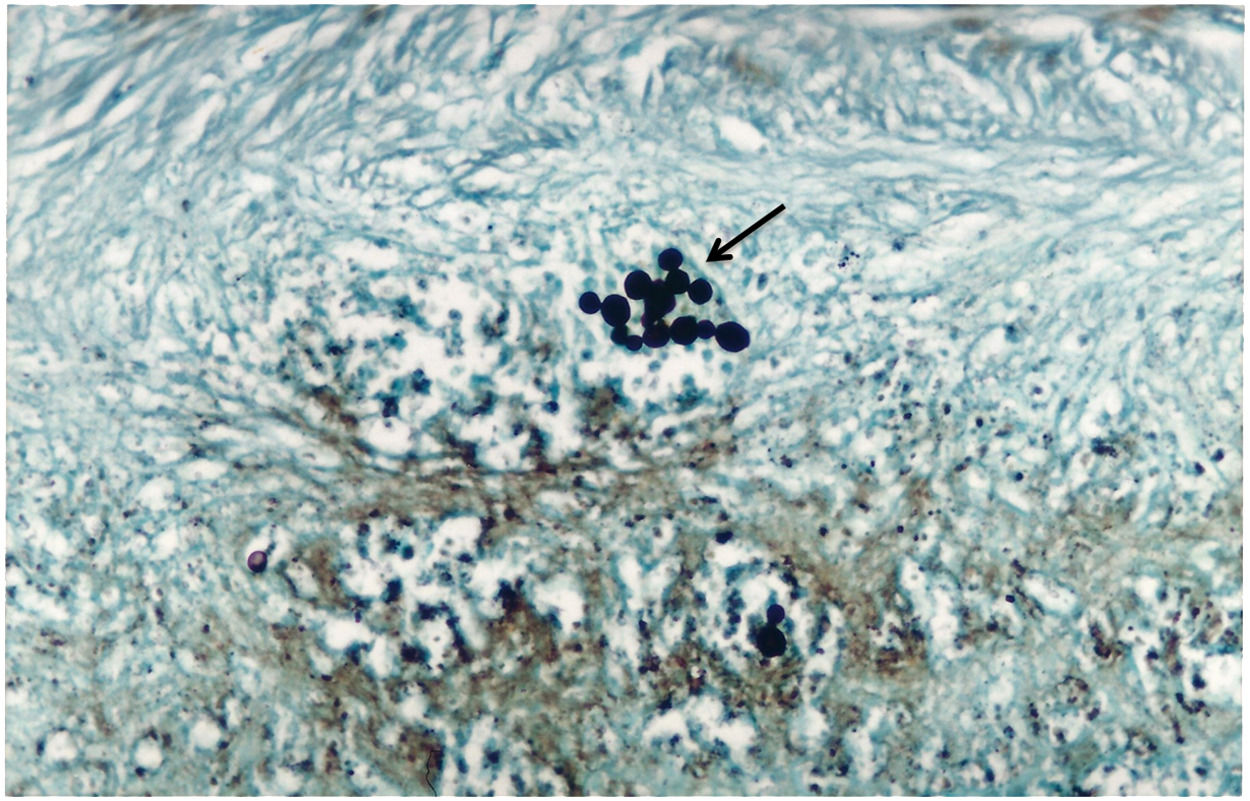
Case report48-years-old Asian, male (E. H.), born in Ribeirão Preto, São Paulo, Brazil, with type I diabetes mellitus and Chronic Kidney Disease (CKD), was undergoing a Continuous Ambulatory Peritoneal Dialysis (CAPD) program. Eighteen months after starting the CAPD program, he began to experience hyporexia, abdominal pain, nausea, and postprandial vomiting, and presented turbid peritoneal fluid. The patient was afebrile, normotensive, pale, and exhibited edema of the lower limbs and diffuse pain upon abdominal palpation, without peritonism. Laboratory tests conducted at the time revealed the following: Creatinine – 13.1 mg/dL; BUN – 36.87 mg/dL; K+ – 3.7 mEq/L; Na+ – 131 mEq/L; Albumin – 2.6 g/dL; Hemoglobin – 13.3 g/dL; Hematocrit – 38%. Peripheral blood examination showed 23,700 leukocytes/mm3 and 87% Polymorphonuclear Neutrophils (PMN). Upon cytological examination, the peritoneal fluid presented 4,900 cells/mm3 and 81% PMN. Cephalothin and tobramycin were used via the intraperitoneal route to initiate treatment. After 48 hours, the peritoneal fluid culture was positive for Escherichia coli, and a new cell count revealed 52 cells/mm3. Since the patient showed improvement, he was discharged from the hospital and instructed to take ciprofloxacin for 14 days. Three days after discharge, he presented with hyporexia, abdominal pain, nausea, postprandial vomiting, and turbidity of the peritoneal fluid. New peritoneal fluid analysis revealed 1,300 cells/mm3 and 90% PMN. Therefore, treatment with cephalothin and tobramycin was reinitiated. The patient developed intestinal subocclusion, followed the next day by increasing peritoneal cellularity (9,200 cells/mm3) associated with clinical worsening. As a result, ceftazidime was started via the intraperitoneal route and vancomycin intravenously. Although peritoneal fluid cellularity remained between 3,000 and 6,000 cells/mm3, with predominant PMN, the patient showed clinical improvement. Three days later, the direct mycological examination of the peritoneal fluid revealed yeasts with morphological aspects suggestive of Paracoccidioides spp. Three subsequent peritoneal fluid samples were again obtained, exhibiting the same finding. A search for anti-Paracoccidioides spp. antibodies in serum by Counter Immunoelectrophoresis (CIEP)8 revealed a titer of 1:16. Based on these findings, the patient was diagnosed with peritonitis caused by PCM. The patient was treated with Sulfamethoxazole-Trimethoprim (SMX-TMP) (1600 mg/320 mg/day) and discontinued the other antimicrobial agents. Radiological examination of the chest and abdomen, abdominal ultrasonography, and abdomen computed tomography were regular. Paracoccidioides spp. was subsequently isolated in a Sabouraud cultures of peritoneal fluid. After three weeks of treatment, the patient showed clinical improvement, and a reduction in peritoneal fluid cellularity (<200 cells/mm3) and was therefore, discharged from the hospital while taking SMX-TMP (1600 mg/320 mg dose/day) and undergoing CAPD. Ten days after discharge and approximately one month after the beginning of treatment with SMX/TMP, the patient was readmitted with a clinical presentation of abdominal pain and turbid peritoneal fluid (15,000 cells/mm3; 81% PMN). Peritoneal fluid cultures were negative for bacteria and fungi, although the direct search for Paracoccidioides spp. was not performed at the time. The patient was treated with vancomycin for 15 days (1,000 mg IV every 5 days), ciprofloxacin for 4 days (400 mg/day IV), metronidazole for 5 days (800 mg/day PO), fluconazole for 3 days (200 mg/day IV), and SMX/TMP (1600 mg/320 mg dose/day) for 61 days. Unfortunately, the patient died 21 days later due to septic shock. The autopsy revealed diffuse fibrinous peritonitis. Microscopic analysis of the peritoneum showed focal points of granulomatous inflammation containing fungal structures, which presented thick walls and multiple budding suggestive of Paracoccidioides spp., in the staining of sections with Hematoxylin-Eosin (HE) and Gomori's Methenamine Silver (GMS) (Figs. 1 and 2, respectively). There was no involvement of the lungs, liver parenchyma (except the hepatic capsule), abdominal lymph nodes, or spleen.
Despite substantial advances in Peritoneal Dialysis (PD) as a renal replacement modality, peritonitis is the most common and severe complication of CAPD and is a significant cause of hospitalization, morbidity, technique failure, and mortality.9 Repeated episodes eventually force the patient to discontinue dialysis modality.10 The primary etiology is bacterial (Gram-positive or Gram-negative), followed by fungal causes.
Fungal peritonitis is severe, albeit infrequent.11 It accounts for 2–13% of cases and is associated with high hospitalization rates, catheter removal, permanent transfer to hemodialysis, and death, with mortality rates reaching 20–30%.12Candida spp. represents 70–90% of cases of fungal peritonitis in adults. Filamentous fungi such as Aspergillus, Penicillium, and other yeasts are much less common, accounting for around 10% of cases. Although, Candida albicans is still predominantly the only pathogen in most centers, four other major Candida species (C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei) have become increasingly recognized causes of fungal peritonitis.12 Another causative agent of fungal peritonitis is Histoplasma capsulatum, a rare condition associated with high morbidity and mortality. Given its lack of specific symptoms and signs, differentiating this type of peritonitis from bacterial peritonitis can be challenging. Few case reports of Histoplasma capsulatum peritonitis in CAPD patients have been published over the past three decades.13 Patients with the acute/subacute form of PCM may present secondary involvement in the peritoneal cavity. Still, no earlier information on peritonitis due to Paracoccidioides spp. was found to be associated with CAPD.
Among the risk factors of fungal peritonitis, most reports are related to the recent use of antibiotics and changes in normal intestinal flora.14 The signs and symptoms are similar to bacterial peritonitis and are almost always preceded by this disease.12 One of the therapeutic options for fungal peritonitis is the administration of antifungal agents and catheter removal. Some studies have suggested removing the catheter when there is no improvement in peritoneal fluid cellularity 48 hours after the beginning of treatment. However, in cases of more severe peritonitis, this alternative increases the risk of peritoneal sclerosis, forcing the patient to change the adopted dialysis modality.12
The patient in question was immunosuppressed due to CKD associated with severe protein-calorie malnutrition. The probable site of primary infection by Paracoccidioides spp. was the peritoneal cavity and the peritoneal membrane, with no apparent involvement of lymph nodes or intra-abdominal parenchymatous organs and no pulmonary involvement. Alternatively, immunodepression could be implied in the reactivation of quiescent abdominal lesions of an old infection by Paracoccidioides spp., developing an opportunistic PCM in the patient. The diagnosis was based on the detection of forms suggestive of the fungus upon direct examination of a peritoneal fluid smear, isolation of the fungus in the culture, and the presence of anti-Paracoccidioides serum antibodies. The simplest method for diagnosing this mycosis is direct visualization of the fungus. Its characteristic morphology and mode of reproduction reveals rounded cells ranging from 2 to 40 mm in size, isolated or grouped, presenting a refringent double wall. The buds can be single or multiple, and the mother cell surrounded by the “buds” is frequently visualized with a characteristic “helm wheel” aspect.6 The most used serological tests to detect specific antibodies are Double Immunodiffusion (DID), CIEP, and Enzyme-Linked Immunosorbent Assay (ELISA). DID is easy to perform, low cost, and has 100% specificity and 65–100% sensitivity. The specificity of CIEP is comparable to that of DID, with 80–100% sensitivity, and titers ≥1:16 suggest active disease.
Based on the usual recommendations for treating this deep mycosis, we opted for clinical treatment with SMX/TMP.6 Because of the good initial response to the scheme used, we left the patient on peritoneal dialysis because of the difficulty in vascular access. The patient's clinical worsening was probably due to the severity of his general conditions and associated bacterial infections. The microlesion of the intestinal wall caused by Paracoccidioides spp. likely facilitated the contamination of the peritoneal cavity by bacteria from the intestinal microbiota.
In conclusion, Paracoccidioides spp. peritonitis is a unique condition and, like Paracoccidioides spp. in other organs, is associated with high morbidity and mortality. In our case, the diagnosis was confirmed through direct mycological examination of the peritoneal fluid, which revealed the presence of fungi with a morphological aspect suggestive of Paracoccidioides spp. This case report describes the first time a patient undergoing CAPD had complications of peritonitis due to opportunistic PCM.
FundingThis study did not receive any specific grant from funding agencies in the public, commercial, or nonprofit sectors.