To evaluate whether patient age has a significant impact on mefloquine concentrations in the plasma and erythrocytes over the course of treatment for uncomplicated falciparum malaria.
MethodsA total of 20 children aged between 8 and 11 years and 20 adult males aged between 22 and 41 years with uncomplicated falciparum malaria were enrolled in the study. Mefloquine was administered to patients in both age groups at a dose of 20mgkg−1. The steady-state drug concentrations were measured by reversed-phase high performance liquid chromatography.
ResultsAll patients had an undetectable mefloquine concentration on day 0. In adults, the plasma mefloquine concentrations ranged from 770 to 2930ngmL−1 and the erythrocyte concentrations ranged from 2000 to 6030ngmL−1. In children, plasma mefloquine concentrations ranged from 881 to 3300ngmL−1 and erythrocyte concentrations ranged from 3000 to 4920ngmL−1. There was no significant correlation between mefloquine concentrations in the plasma and erythrocytes in either adults or children.
ConclusionIn the present study, we observed no effect of patient age on the steady-state concentrations of mefloquine in the plasma and erythrocytes. We found that the mefloquine concentration in the erythrocytes was approximately 2.8-times higher than in the plasma. There were no significant correlations between mefloquine concentrations in the erythrocytes and plasma for either age group.
Mefloquine is a quinoline methanol compound which is effective against the asexual blood stages of Plasmodium falciparum. It was first introduced in 1984 for clinical use in Thailand. Currently the drug is used for the treatment of the acute phase of malaria, either alone or in combination with artesunate, or as preventative monotherapy for individuals traveling to endemic areas.1,2
The exact mechanism of action of mefloquine remains unknown. However, it probably induces morphological changes in the early ring stages of P. falciparum to inhibit haem polymerization. Administration of mefloquine results in formation of the mefloquine-ferriprotoporphyrin IX complex, which is toxic to the parasite.3,4 The concentration of mefloquine within erythrocytes is one of the major determinants of its therapeutic efficacy. Thus, it is likely that a change in exposure to mefloquine would alter the concentration of the drug within red blood cells, and consequently, the treatment outcomes.5,6 Studies investigating the pharmacokinetics of mefloquine have demonstrated that the concentration of the drug in blood components is influenced by several factors including gender, body weight, disease severity, parasitemia at hospital admission, drug malabsorption, vomiting, drug formulation, and ingestion of certain foods.6,7
It is plausible that the patient's age may also affect the concentration of mefloquine in the blood components. A higher rate of therapeutic failure has been reported in children compared to adults. This was found to be associated with reduced concentrations of mefloquine in the blood as a result of malabsorption, diarrhea or vomiting.6,7 As children are at higher risk for cerebral malaria, the efficacy of antimalarial drugs needs to be carefully evaluated. However, there is limited data on the effect of age on the concentration of mefloquine within erythrocytes. Therefore, the aim of this study was to determine whether the patient's age has an effect on the concentration of mefloquine in the plasma and erythrocytes during treatment of the acute phase of uncomplicated falciparum malaria.
Materials and methodsEthical statementThe study was approved by the Ethical Committee of Associação Educacional da Amazônia-SEAMA (079/08). All adult patients and caretakers or guardians of the children enrolled in the study were informed about the goals of the study, as well as the risks and benefits, before giving written informed consent prior to entering the study according to the National Committee of Ethics in Research (CONEP; 196/96). The children received verbal instructions about the study, and the collection of blood was performed in the presence of their caretakers or guardians.
Study location and patientsThe study was carried out at the Reference Center for Tropical Disease in Macapa (Brazil), which is located in an area of low malarial transmission alongside the Amazon River. A total of 20 male children and 20 male adults with slide-confirmed P. falciparum infection were enrolled in the study. The exclusion criteria were signs or symptoms of severe or mixed malaria (jaundice, renal impairment, severe anemia, or an altered level of consciousness), parasitemia above 5%, a known hypersensitivity or allergy to mefloquine, individuals that had used antimalarial drugs in the four weeks prior to admission, or individuals with a known history of psychiatric disorders.
Clinical and laboratory assessmentAll patients underwent clinical and laboratory evaluation at admission on day 0 (D0) and on days 3, 7, 14, 28 and 42. A complete blood count was performed on D0 and day 3 (D3). Blood samples for the measurement of mefloquine were collected on D0 and D3.
Mefloquine treatmentEach participant received multiple oral doses of 250mg mefloquine base tablets (Farmanguinhos; Brazilian Health Office) on days 1 and 2.1,8 The dose was adjusted for the weight of the patient to receive a total dose of 20mg/kg. The drug was administered with water 30min after a typical Amazonian breakfast. No additional food or drink was provided for 2h following administration of the drug. In case of vomiting within 2h of mefloquine administration, the patients were carefully supervised by clinical staff.
Blood sample collection and measurement of mefloquineVenous blood samples (4mL) were collected from each patient in lithium heparin tubes on D0 and 24h after the last dose of mefloquine (D3). After collection, the samples (4mL) were immediately centrifuged at 500×g for 10min at 4°C for the separation of plasma. A cell wash buffer was added to the erythrocyte pellet, which was then centrifuged at 500×g for 10min. This procedure was repeated five times for the separation of erythrocytes. The samples were stored at −80°C until analysis.
A reversed-phase high performance liquid chromatography (HPLC) system with ultraviolet detection (ProStar; Varian, Walnut, CA) was used for the analysis of mefloquine after liquid-liquid extraction of the drug from the blood components with methyl tert-butyl ether at pH 4.0. The separation was carried out using a reversed-phase column (ODS C18; 4.6mm×250mm, 5μm internal diameter; Supelco Inc., Bellefonte, PA) with a mobile phase composed of acetonitrile: phosphate buffer (pH 2.5) with a ratio of 42:58. Quinidine (2.5μg/mL) was used as the internal standard. The within-day and day-to-day coefficients of variation for the laboratory conditions were 6.7% and 8.1%, respectively. The assay was linear from 150 to 5000ng/mL and the detection limit was 50ng/mL. The mean recovery was 95%. The stability of blank plasma spiked with mefloquine was 60 days.9
Data analysisThe data are described as the median value and measurement range. The Mann–Whitney U test was used to compare the concentrations of mefloquine in the plasma and erythrocytes for both age groups. The Spearman's correlation coefficient was used to estimate the correlation between the mefloquine concentrations in the erythrocytes and plasma for both age groups. All p-values were two-tailed and p<0.05 was considered to be statistically significant. All statistical analyses were performed with Statistica software (version 7.0; StatSoft Inc., Tulsa, OK).
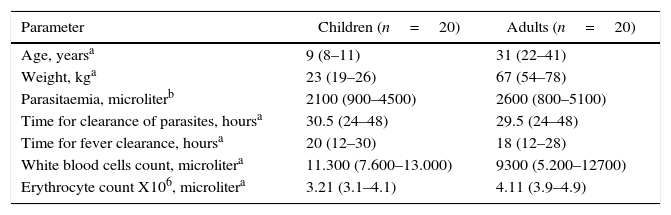
ResultsThe baseline characteristics of all patients are shown in Table 1. The geometric mean parasite density was similar for both age groups. All patients completed the 42-day follow-up period. There was no resurgence of the parasite in the peripheral blood, and all patients showed an adequate response to treatment as demonstrated by complete clearance of parasites from the peripheral blood within three days of starting treatment. The mean parasite clearance times were 30.5h (ranged from 24 to 48h) and 29.5h (ranged from 24 to 48h)h in children and adults, respectively. The mean time for the fever to clear was 20h (ranged from 12 to 30h) in the children and 18h (ranged from 12 to 28h) in the adults. The drug was well tolerated, as only two children experienced slight nausea and dizziness and there was no report of vomiting after drug intake.
Baseline characteristics of patients included in the study.
| Parameter | Children (n=20) | Adults (n=20) |
|---|---|---|
| Age, yearsa | 9 (8–11) | 31 (22–41) |
| Weight, kga | 23 (19–26) | 67 (54–78) |
| Parasitaemia, microliterb | 2100 (900–4500) | 2600 (800–5100) |
| Time for clearance of parasites, hoursa | 30.5 (24–48) | 29.5 (24–48) |
| Time for fever clearance, hoursa | 20 (12–30) | 18 (12–28) |
| White blood cells count, microlitera | 11.300 (7.600–13.000) | 9300 (5.200–12700) |
| Erythrocyte count X106, microlitera | 3.21 (3.1–4.1) | 4.11 (3.9–4.9) |
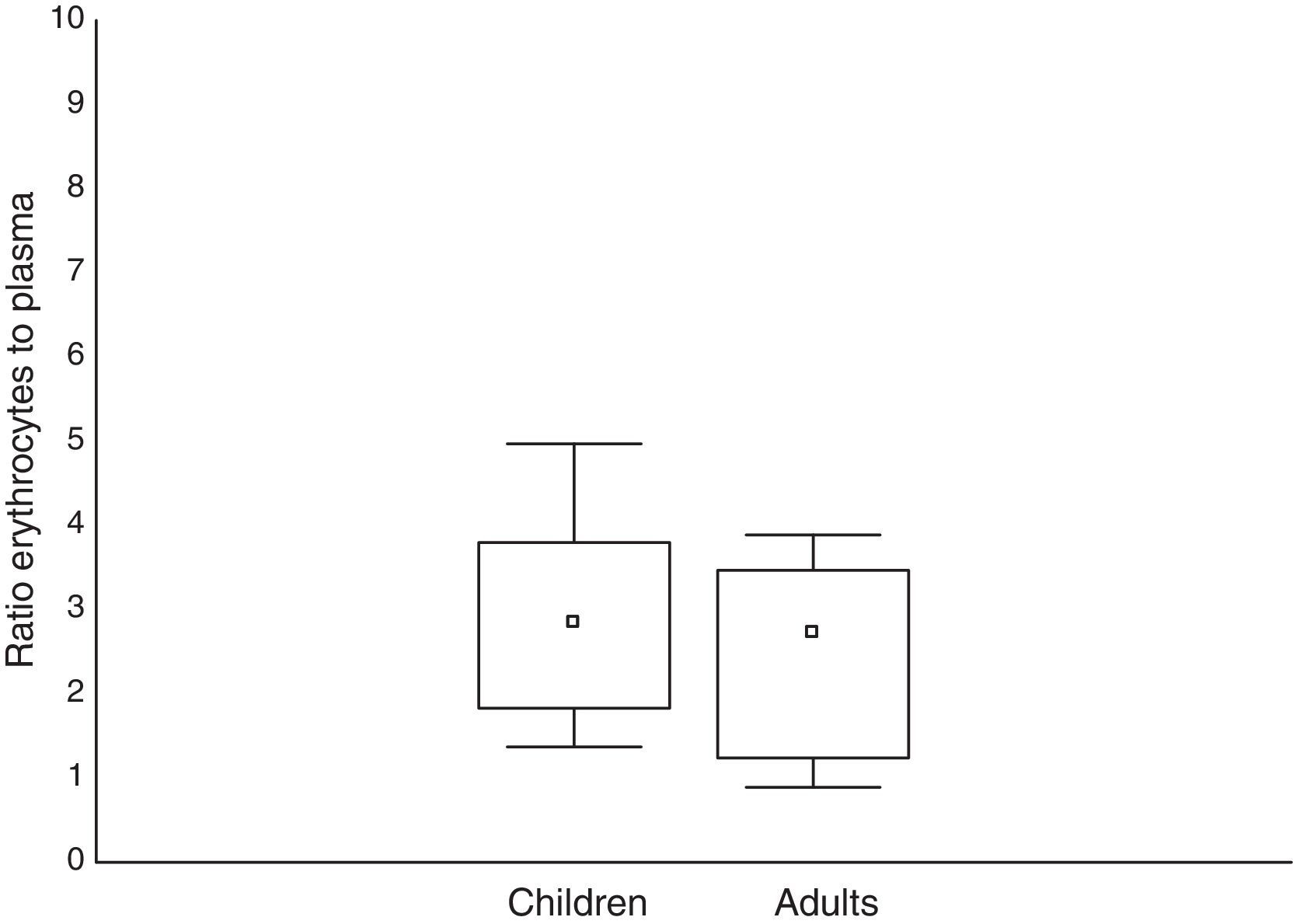
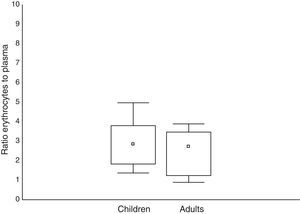
All patients had no measurable concentration of mefloquine on D0. In adults, the concentration of mefloquine in the plasma ranged from 770 to 2930ngmL−1 and from 2000 to 6030ngmL−1 in the erythrocytes. The erythrocyte-to-plasma concentration ratio ranged from 0.89 to 3.89. In children, the concentration of mefloquine in the plasma ranged from 881 to 3300ngmL−1 and from 3000 to 4920ngmL−1 in the erythrocytes. The erythrocyte-to-plasma concentration ratio ranged from 1.37 to 4.97 in the children. The mefloquine concentrations were similar in the adults and children in both the plasma (U=210; p=0.60) and erythrocyte (U=228; p=0.94) concentrations. A similar result was found for the erythrocyte-to-plasma concentration ratio between the different age groups (U=192; p=0.34). The mefloquine concentrations found in the erythrocytes and plasma for both age groups are presented in Table 2, and the erythrocyte-to-plasma concentration ratios are presented in Fig. 1. There were no significant correlations between the concentration of mefloquine in the erythrocytes and plasma of adults (s=0.102; p=0.32) or children (s=0.215; p=0.21).
Concentrations of mefloquine determined in the plasma and erythrocytes of children and adults with uncomplicated falciparum malaria.
This study investigated the effect of age on the concentration of mefloquine in the plasma and erythrocytes of patients with uncomplicated falciparum malaria in the Amazon basin. Drug administration and the occurrence of vomiting and diarrhea were carefully monitored by the clinical research staff.
This is the first time that plasma mefloquine concentrations have been measured in a riverside population from the Brazilian Amazon basin. The concentration of mefloquine found in adult patients was similar to that reported in previous studies which investigated healthy Caucasian volunteers and patients with uncomplicated falciparum malaria from Thailand and Peru.10–12 In children, the median concentration of mefloquine in the plasma was similar to that obtained in a study including Karen children.13 Furthermore, there were no significant differences in mefloquine concentrations between children and adults. This is consistent with previous studies which investigated and compared the pharmacokinetic parameters of mefloquine in patients with uncomplicated falciparum malaria of different age groups. These studies only found an effect of body weight on drug clearance in children aged from 6 to 24 months when compared to older children (aged from 5 to 12 years), which were similar to adult patients.14–17 In addition, the concentrations of mefloquine in all patients were above 500ngmL−1, which was associated with high rates of treatment efficacy.15,18
In the erythrocytes, the mefloquine concentrations were also similar for both age groups. Furthermore, the median concentration of mefloquine in the erythrocytes was about 2.8-times higher than in the plasma for both age groups. This is consistent with an in vitro study which reported a relatively constant distribution of mefloquine in the erythrocytes and plasma, with a ratio of approximately 2:1, suggesting the potential accumulation of mefloquine within the erythrocytes.19 Despite the high binding of mefloquine to plasma proteins, it passively crosses the membrane and binds to the membrane phospholipids and ferriprotoporphirin IX, forming a mefloquine-ferriprotoporphirin IX complex which is toxic to the Plasmodium. This complex is responsible for the high drug concentration observed in parasitized erythrocytes, which is approximately 3- to 4-times higher than non-parasitized erythrocytes.19–23
On the other hand, the findings of the study were not consistent with a previous study on the distribution of mefloquine in the blood components of patients with uncomplicated falciparum malaria in Thailand, which reported similar concentrations of mefloquine in the whole blood and plasma. Furthermore, another study in the same population group which investigated the influence of gender on mefloquine concentrations in blood components reported drug accumulation in the plasma, with the plasma-to-erythrocytes ratio ranging from 1.59 to 3.75. There are several possible explanations for the discrepancies between studies, including parasitemia at admission, disease severity, mefloquine formulation, or the time between drug administration and blood sampling.11,24,10
There were no significant correlations between the concentration of mefloquine in the plasma and erythrocytes for either age group. This may be due to the affinity of the drug to other blood components, rather than the plasma or erythrocytes. This is in agreement with two studies which evaluated the distribution of mefloquine in the blood components of Thai patients with acute uncomplicated falciparum malaria and in healthy Caucasians. Both studies reported that the mefloquine concentrations in blood components was highest in white blood cells, followed by platelets, plasma, serum, whole blood, and lowest in erythrocytes.24,10
A potential limitation of the present study is that only one blood sample was collected from each patient to estimate mefloquine concentrations in both blood components. However, measuring anti-malarial drugs with a longer half-life in the steady state appears to provide reliable data which can be used to estimate the exposure of patients to these medicines.25
ConclusionsIn the present study, we observed no effect of patient age on the steady-state concentrations of mefloquine in plasma and in erythrocytes. In addition, the concentration of the drug in erythrocytes was approximately 2.8-times higher than in plasma. Finally, there were no significant correlations observed between the concentrations of mefloquine in the erythrocytes and the plasma for either age group.
Conflicts of interestThe authors declare no conflicts of interest.